

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sialidase A (Streptococcus pneumoniae) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 9.0 | n/a |
UniversitÓ degli Studi di Siena | Assay Description Briefly, the reaction mixtures containing the substrates and up to 100 nM sialidase were incubated at 37░C and stopped by the addition of 0.5 M Na2CO... | BMC Biochem 13: 19 (2012) Article DOI: 10.1186/1471-2091-13-19 BindingDB Entry DOI: 10.7270/Q2959GDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 9.0 | n/a |
UniversitÓ degli Studi di Siena | Assay Description Briefly, the reaction mixtures containing the substrates and up to 100 nM sialidase were incubated at 37░C and stopped by the addition of 0.5 M Na2CO... | BMC Biochem 13: 19 (2012) Article DOI: 10.1186/1471-2091-13-19 BindingDB Entry DOI: 10.7270/Q2959GDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase NanB (Streptococcus pneumoniae) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 9.0 | n/a |
UniversitÓ degli Studi di Siena | Assay Description Briefly, the reaction mixtures containing the substrates and up to 100 nM sialidase were incubated at 37░C and stopped by the addition of 0.5 M Na2CO... | BMC Biochem 13: 19 (2012) Article DOI: 10.1186/1471-2091-13-19 BindingDB Entry DOI: 10.7270/Q2959GDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM4934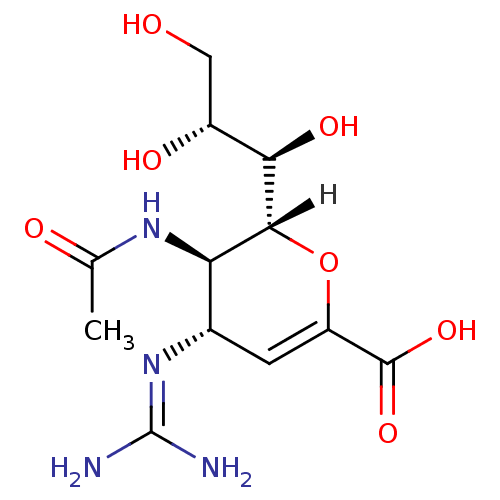 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[(1R,2R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 9.0 | n/a |
UniversitÓ degli Studi di Siena | Assay Description Briefly, the reaction mixtures containing the substrates and up to 100 nM sialidase were incubated at 37░C and stopped by the addition of 0.5 M Na2CO... | BMC Biochem 13: 19 (2012) Article DOI: 10.1186/1471-2091-13-19 BindingDB Entry DOI: 10.7270/Q2959GDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| FMN_red domain-containing protein (Streptococcus pneumoniae) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.01E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 9.0 | n/a |
UniversitÓ degli Studi di Siena | Assay Description Briefly, the reaction mixtures containing the substrates and up to 100 nM sialidase were incubated at 37░C and stopped by the addition of 0.5 M Na2CO... | BMC Biochem 13: 19 (2012) Article DOI: 10.1186/1471-2091-13-19 BindingDB Entry DOI: 10.7270/Q2959GDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||