 Found 67 hits with Last Name = 'bai' and Initial = 'lg'
Found 67 hits with Last Name = 'bai' and Initial = 'lg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50079362
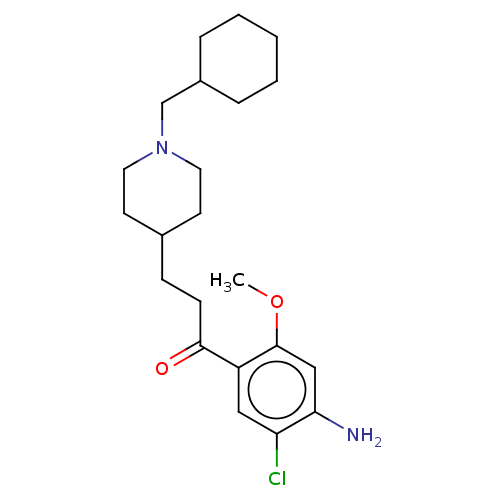
(CHEMBL3417009 | US9663465, 9)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(CC2CCCCC2)CC1 Show InChI InChI=1S/C22H33ClN2O2/c1-27-22-14-20(24)19(23)13-18(22)21(26)8-7-16-9-11-25(12-10-16)15-17-5-3-2-4-6-17/h13-14,16-17H,2-12,15,24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50592632
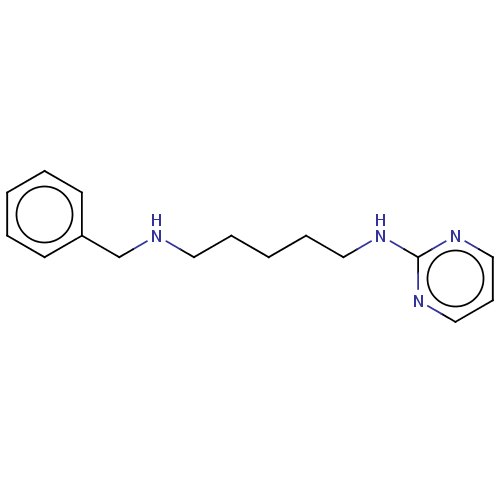
(CHEMBL5190650) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50079362

(CHEMBL3417009 | US9663465, 9)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(CC2CCCCC2)CC1 Show InChI InChI=1S/C22H33ClN2O2/c1-27-22-14-20(24)19(23)13-18(22)21(26)8-7-16-9-11-25(12-10-16)15-17-5-3-2-4-6-17/h13-14,16-17H,2-12,15,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50544937
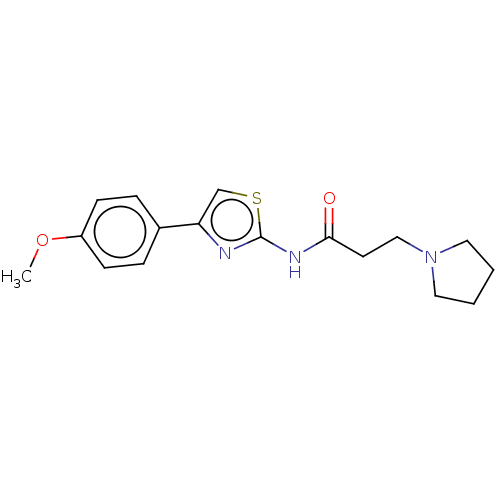
(CHEMBL513063)Show InChI InChI=1S/C17H21N3O2S/c1-22-14-6-4-13(5-7-14)15-12-23-17(18-15)19-16(21)8-11-20-9-2-3-10-20/h4-7,12H,2-3,8-11H2,1H3,(H,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544927
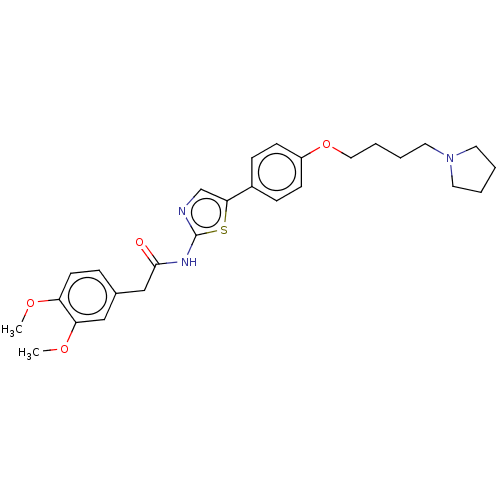
(CHEMBL4648831)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCN3CCCC3)cc2)cc1OC Show InChI InChI=1S/C27H33N3O4S/c1-32-23-12-7-20(17-24(23)33-2)18-26(31)29-27-28-19-25(35-27)21-8-10-22(11-9-21)34-16-6-5-15-30-13-3-4-14-30/h7-12,17,19H,3-6,13-16,18H2,1-2H3,(H,28,29,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50544925
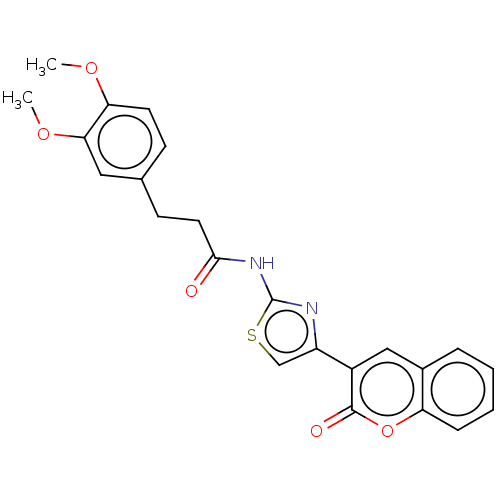
(CHEMBL4645659)Show SMILES COc1ccc(CCC(=O)Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1OC Show InChI InChI=1S/C23H20N2O5S/c1-28-19-9-7-14(11-20(19)29-2)8-10-21(26)25-23-24-17(13-31-23)16-12-15-5-3-4-6-18(15)30-22(16)27/h3-7,9,11-13H,8,10H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodide as substrate incubated for 15 mins by Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592626
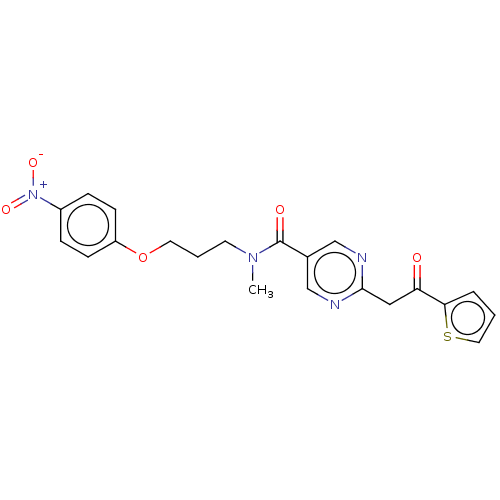
(CHEMBL5187324)Show SMILES CN(CCCOc1ccc(cc1)[N+]([O-])=O)C(=O)c1cnc(CC(=O)c2cccs2)nc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544936
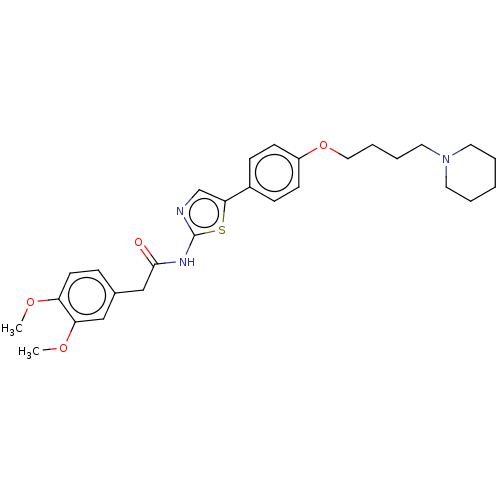
(CHEMBL4649371)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCN3CCCCC3)cc2)cc1OC Show InChI InChI=1S/C28H35N3O4S/c1-33-24-13-8-21(18-25(24)34-2)19-27(32)30-28-29-20-26(36-28)22-9-11-23(12-10-22)35-17-7-6-16-31-14-4-3-5-15-31/h8-13,18,20H,3-7,14-17,19H2,1-2H3,(H,29,30,32) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592624
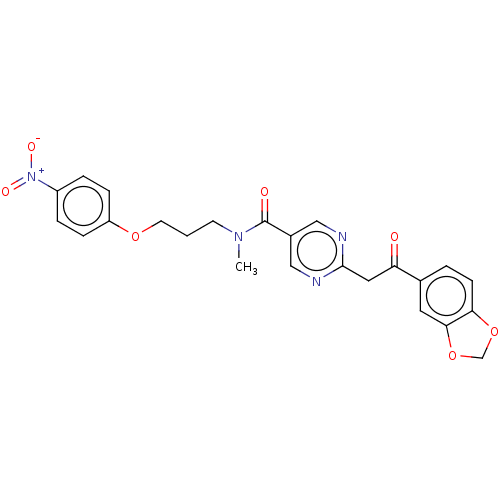
(CHEMBL5208032)Show SMILES CN(CCCOc1ccc(cc1)[N+]([O-])=O)C(=O)c1cnc(CC(=O)c2ccc3OCOc3c2)nc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592622
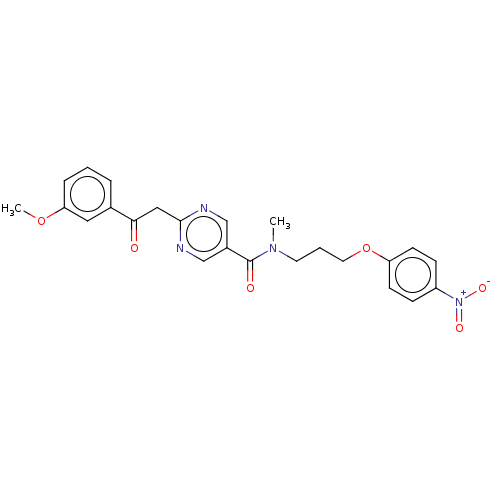
(CHEMBL5185065)Show SMILES COc1cccc(c1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)[N+]([O-])=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544926
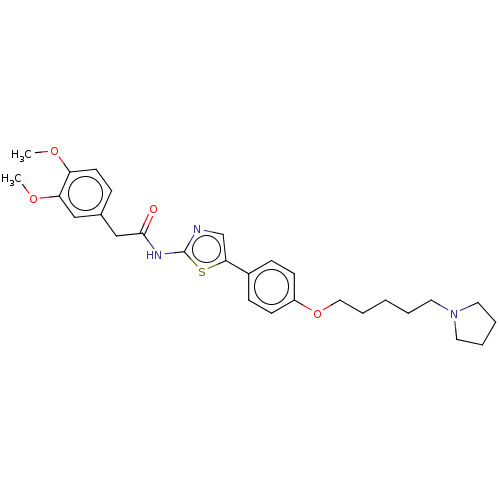
(CHEMBL4644714)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCCN3CCCC3)cc2)cc1OC Show InChI InChI=1S/C28H35N3O4S/c1-33-24-13-8-21(18-25(24)34-2)19-27(32)30-28-29-20-26(36-28)22-9-11-23(12-10-22)35-17-7-3-4-14-31-15-5-6-16-31/h8-13,18,20H,3-7,14-17,19H2,1-2H3,(H,29,30,32) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592631
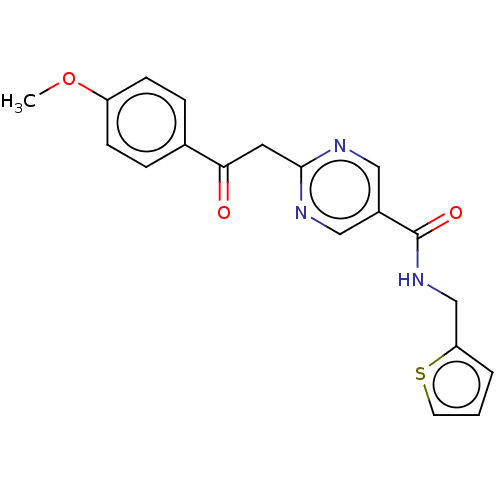
(CHEMBL5205468) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592628
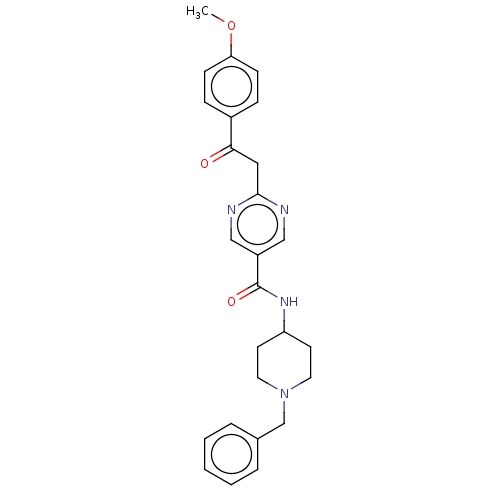
(CHEMBL5185843)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)NC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592625
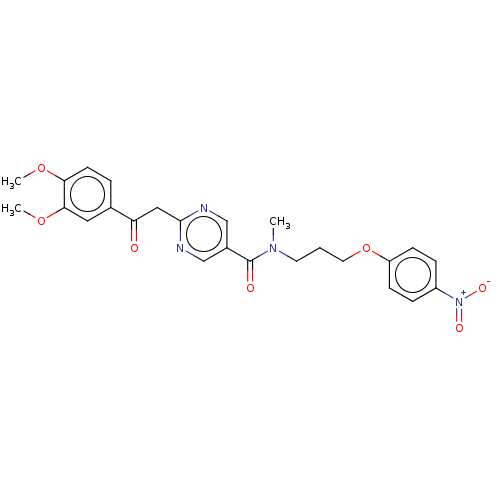
(CHEMBL5203792)Show SMILES COc1ccc(cc1OC)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592622

(CHEMBL5185065)Show SMILES COc1cccc(c1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544933
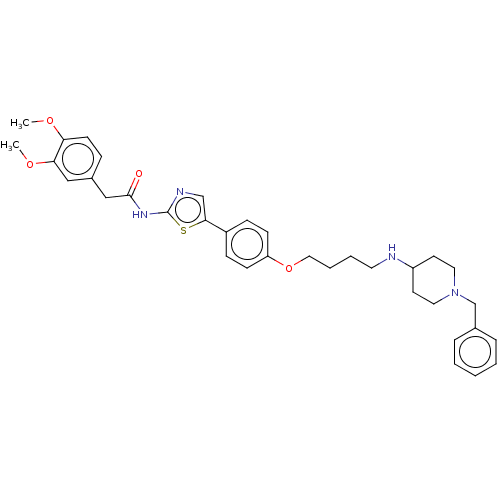
(CHEMBL4646119)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCNC3CCN(Cc4ccccc4)CC3)cc2)cc1OC Show InChI InChI=1S/C35H42N4O4S/c1-41-31-15-10-27(22-32(31)42-2)23-34(40)38-35-37-24-33(44-35)28-11-13-30(14-12-28)43-21-7-6-18-36-29-16-19-39(20-17-29)25-26-8-4-3-5-9-26/h3-5,8-15,22,24,29,36H,6-7,16-21,23,25H2,1-2H3,(H,37,38,40) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592618
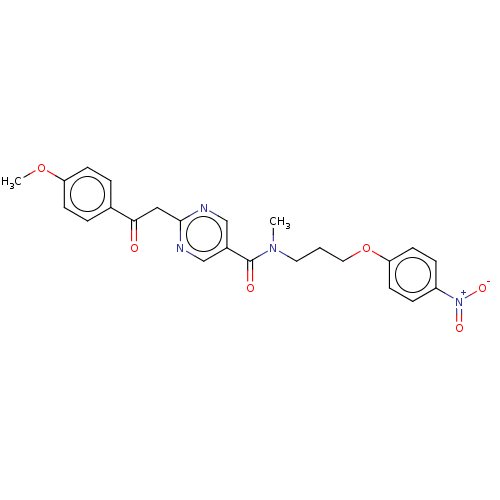
(CHEMBL5172343)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592620
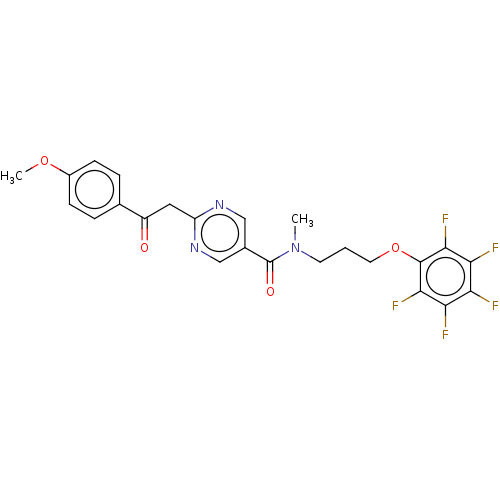
(CHEMBL5186394)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1c(F)c(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50199522
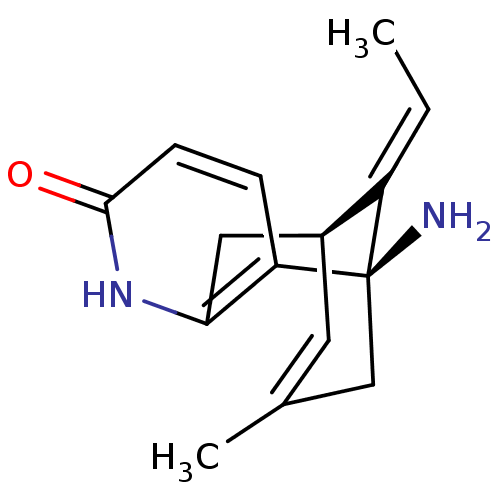
((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50199522

((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544935
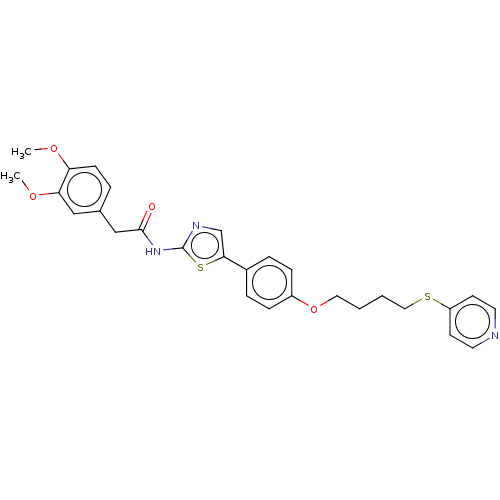
(CHEMBL4638000)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCSc3ccncc3)cc2)cc1OC Show InChI InChI=1S/C28H29N3O4S2/c1-33-24-10-5-20(17-25(24)34-2)18-27(32)31-28-30-19-26(37-28)21-6-8-22(9-7-21)35-15-3-4-16-36-23-11-13-29-14-12-23/h5-14,17,19H,3-4,15-16,18H2,1-2H3,(H,30,31,32) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592629

(CHEMBL5186796)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592625

(CHEMBL5203792)Show SMILES COc1ccc(cc1OC)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)[N+]([O-])=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544934
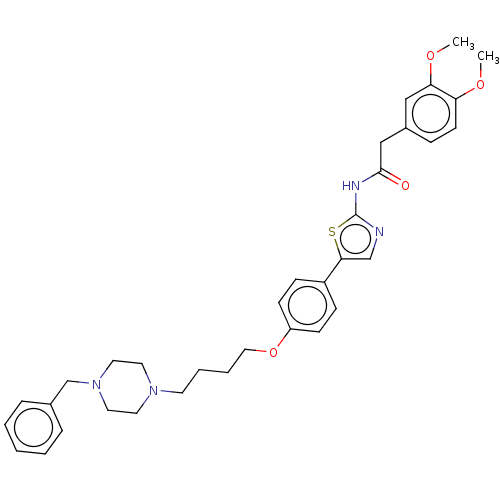
(CHEMBL4645093)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCN3CCN(Cc4ccccc4)CC3)cc2)cc1OC Show InChI InChI=1S/C34H40N4O4S/c1-40-30-15-10-27(22-31(30)41-2)23-33(39)36-34-35-24-32(43-34)28-11-13-29(14-12-28)42-21-7-6-16-37-17-19-38(20-18-37)25-26-8-4-3-5-9-26/h3-5,8-15,22,24H,6-7,16-21,23,25H2,1-2H3,(H,35,36,39) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592621
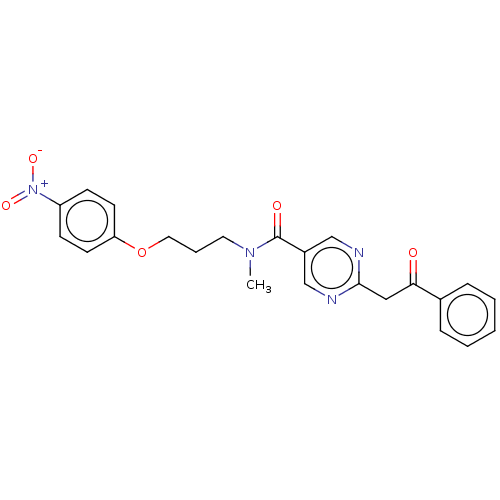
(CHEMBL5204734)Show SMILES CN(CCCOc1ccc(cc1)[N+]([O-])=O)C(=O)c1cnc(CC(=O)c2ccccc2)nc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592619
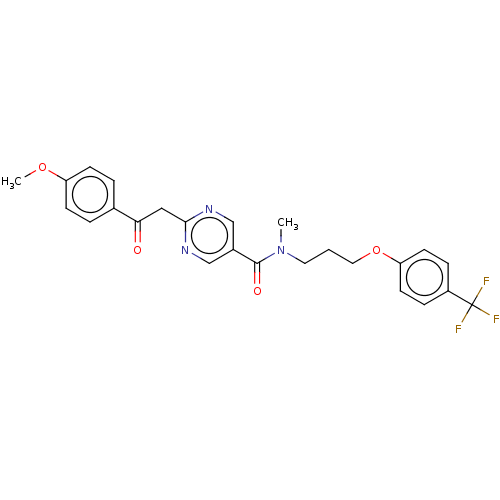
(CHEMBL5171192)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM11682
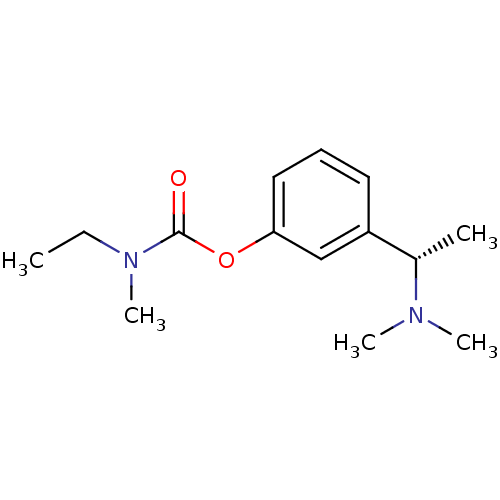
(2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544928
(CHEMBL4645455)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCN3CCCC3)cc2)cc1OC Show InChI InChI=1S/C25H29N3O4S/c1-30-21-10-5-18(15-22(21)31-2)16-24(29)27-25-26-17-23(33-25)19-6-8-20(9-7-19)32-14-13-28-11-3-4-12-28/h5-10,15,17H,3-4,11-14,16H2,1-2H3,(H,26,27,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592623
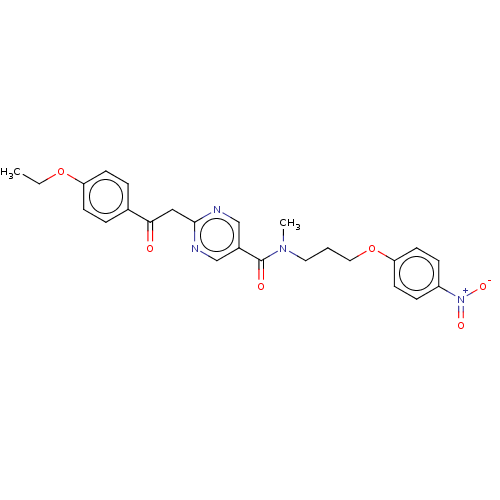
(CHEMBL5171010)Show SMILES CCOc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544931

(CHEMBL4639673)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCN3CCCC3)cc2)cc1OC Show InChI InChI=1S/C26H31N3O4S/c1-31-22-11-6-19(16-23(22)32-2)17-25(30)28-26-27-18-24(34-26)20-7-9-21(10-8-20)33-15-5-14-29-12-3-4-13-29/h6-11,16,18H,3-5,12-15,17H2,1-2H3,(H,27,28,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592623

(CHEMBL5171010)Show SMILES CCOc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)[N+]([O-])=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM50544931

(CHEMBL4639673)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCN3CCCC3)cc2)cc1OC Show InChI InChI=1S/C26H31N3O4S/c1-31-22-11-6-19(16-23(22)32-2)17-25(30)28-26-27-18-24(34-26)20-7-9-21(10-8-20)33-15-5-14-29-12-3-4-13-29/h6-11,16,18H,3-5,12-15,17H2,1-2H3,(H,27,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM50544933

(CHEMBL4646119)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCNC3CCN(Cc4ccccc4)CC3)cc2)cc1OC Show InChI InChI=1S/C35H42N4O4S/c1-41-31-15-10-27(22-32(31)42-2)23-34(40)38-35-37-24-33(44-35)28-11-13-30(14-12-28)43-21-7-6-18-36-29-16-19-39(20-17-29)25-26-8-4-3-5-9-26/h3-5,8-15,22,24,29,36H,6-7,16-21,23,25H2,1-2H3,(H,37,38,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50544929
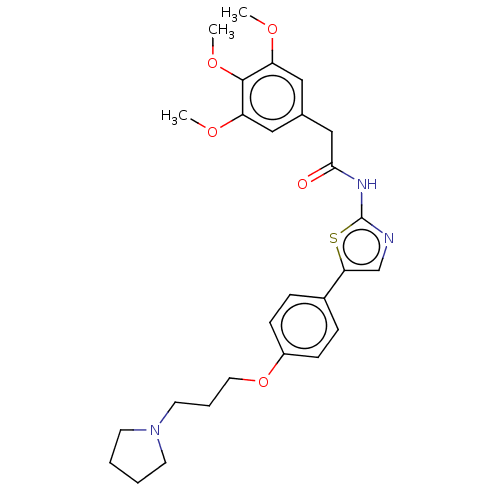
(CHEMBL4638435)Show SMILES COc1cc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCN3CCCC3)cc2)cc(OC)c1OC Show InChI InChI=1S/C27H33N3O5S/c1-32-22-15-19(16-23(33-2)26(22)34-3)17-25(31)29-27-28-18-24(36-27)20-7-9-21(10-8-20)35-14-6-13-30-11-4-5-12-30/h7-10,15-16,18H,4-6,11-14,17H2,1-3H3,(H,28,29,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM50544932
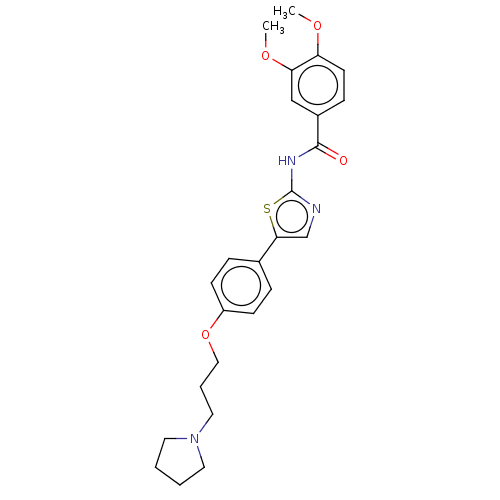
(CHEMBL4634668)Show SMILES COc1ccc(cc1OC)C(=O)Nc1ncc(s1)-c1ccc(OCCCN2CCCC2)cc1 Show InChI InChI=1S/C25H29N3O4S/c1-30-21-11-8-19(16-22(21)31-2)24(29)27-25-26-17-23(33-25)18-6-9-20(10-7-18)32-15-5-14-28-12-3-4-13-28/h6-11,16-17H,3-5,12-15H2,1-2H3,(H,26,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592617
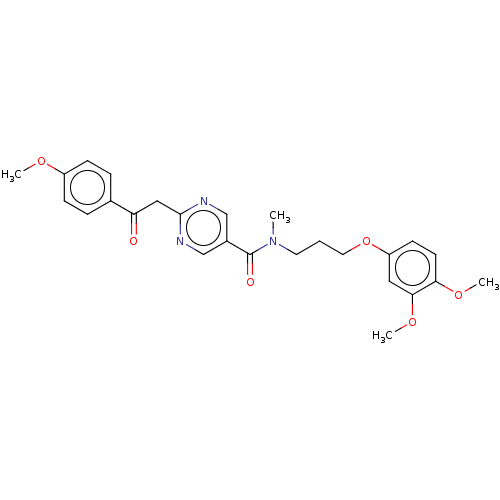
(CHEMBL5193255)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(OC)c(OC)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592616
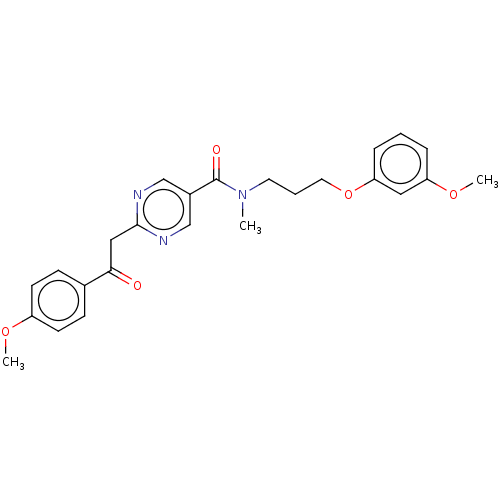
(CHEMBL5186051)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1cccc(OC)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM50544930
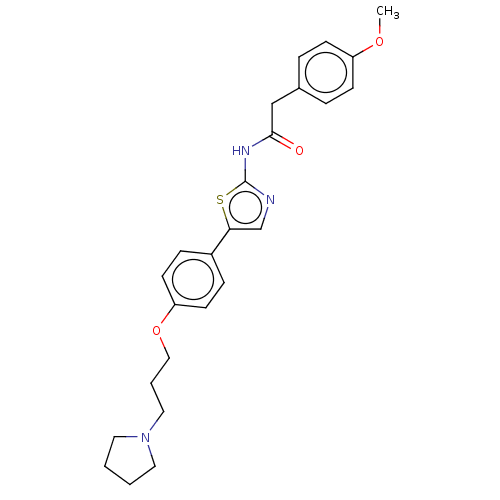
(CHEMBL4640975)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCN3CCCC3)cc2)cc1 Show InChI InChI=1S/C25H29N3O3S/c1-30-21-9-5-19(6-10-21)17-24(29)27-25-26-18-23(32-25)20-7-11-22(12-8-20)31-16-4-15-28-13-2-3-14-28/h5-12,18H,2-4,13-17H2,1H3,(H,26,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592615
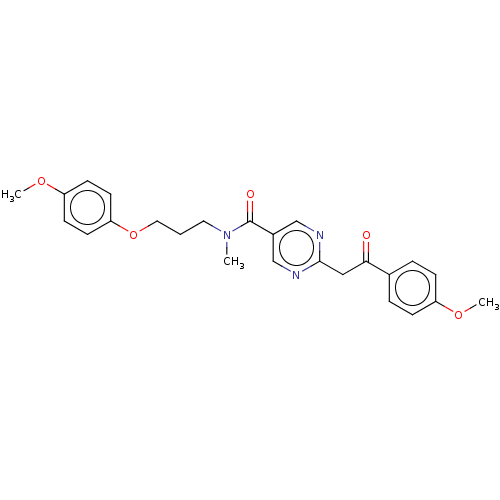
(CHEMBL5196988)Show SMILES COc1ccc(OCCCN(C)C(=O)c2cnc(CC(=O)c3ccc(OC)cc3)nc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM11682

(2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE using acetylcholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592615

(CHEMBL5196988)Show SMILES COc1ccc(OCCCN(C)C(=O)c2cnc(CC(=O)c3ccc(OC)cc3)nc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM50544926

(CHEMBL4644714)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCCN3CCCC3)cc2)cc1OC Show InChI InChI=1S/C28H35N3O4S/c1-33-24-13-8-21(18-25(24)34-2)19-27(32)30-28-29-20-26(36-28)22-9-11-23(12-10-22)35-17-7-3-4-14-31-15-5-6-16-31/h8-13,18,20H,3-7,14-17,19H2,1-2H3,(H,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592621

(CHEMBL5204734)Show SMILES CN(CCCOc1ccc(cc1)[N+]([O-])=O)C(=O)c1cnc(CC(=O)c2ccccc2)nc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592626

(CHEMBL5187324)Show SMILES CN(CCCOc1ccc(cc1)[N+]([O-])=O)C(=O)c1cnc(CC(=O)c2cccs2)nc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592627
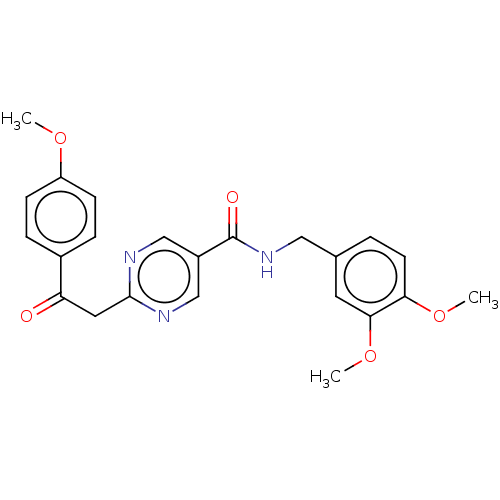
(CHEMBL5171268)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)NCc1ccc(OC)c(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM50544927

(CHEMBL4648831)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCN3CCCC3)cc2)cc1OC Show InChI InChI=1S/C27H33N3O4S/c1-32-23-12-7-20(17-24(23)33-2)18-26(31)29-27-28-19-25(35-27)21-8-10-22(11-9-21)34-16-6-5-15-30-13-3-4-14-30/h7-12,17,19H,3-6,13-16,18H2,1-2H3,(H,28,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50592618

(CHEMBL5172343)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N(C)CCCOc1ccc(cc1)[N+]([O-])=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM50544935

(CHEMBL4638000)Show SMILES COc1ccc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCCSc3ccncc3)cc2)cc1OC Show InChI InChI=1S/C28H29N3O4S2/c1-33-24-10-5-20(17-25(24)34-2)18-27(32)31-28-30-19-26(37-28)21-6-8-22(9-7-21)35-15-3-4-16-36-23-11-13-29-14-12-23/h5-14,17,19H,3-4,15-16,18H2,1-2H3,(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Canis lupus familiaris) | BDBM50544929

(CHEMBL4638435)Show SMILES COc1cc(CC(=O)Nc2ncc(s2)-c2ccc(OCCCN3CCCC3)cc2)cc(OC)c1OC Show InChI InChI=1S/C27H33N3O5S/c1-32-22-15-19(16-23(33-2)26(22)34-3)17-25(31)29-27-28-18-24(36-27)20-7-9-21(10-8-20)35-14-6-13-30-11-4-5-12-30/h7-10,15-16,18H,4-6,11-14,17H2,1-3H3,(H,28,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University
Curated by ChEMBL
| Assay Description
Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126985
BindingDB Entry DOI: 10.7270/Q2QR51QF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50592629

(CHEMBL5186796)Show SMILES COc1ccc(cc1)C(=O)Cc1ncc(cn1)C(=O)N1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128873
BindingDB Entry DOI: 10.7270/Q2TT4VX9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data