

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-3 (Homo sapiens (Human)) | BDBM10246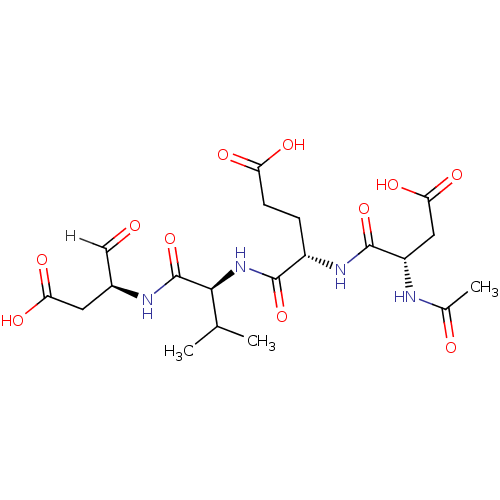 ((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10358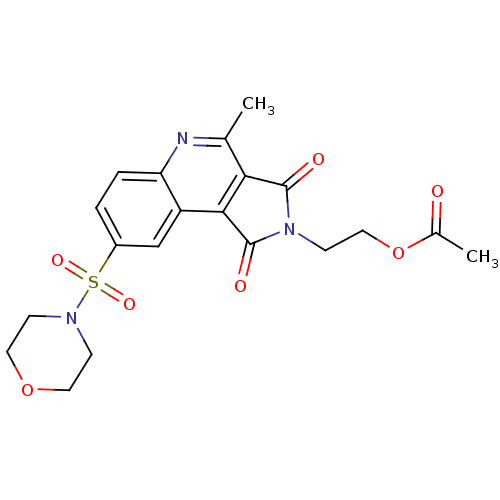 (2-[7-methyl-12-(morpholine-4-sulfonyl)-3,5-dioxo-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10360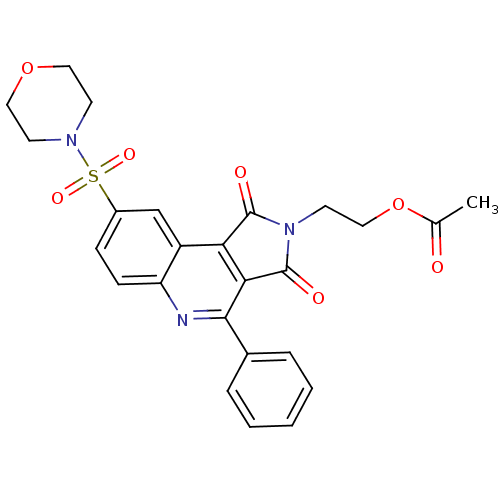 (2-[12-(morpholine-4-sulfonyl)-3,5-dioxo-7-phenyl-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10362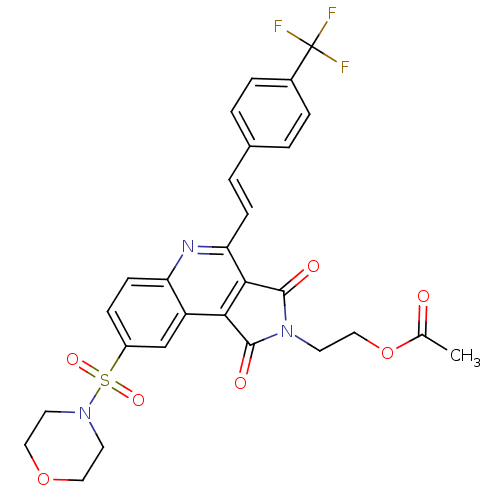 (2-[12-(morpholine-4-sulfonyl)-3,5-dioxo-7-[(E)-2-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10374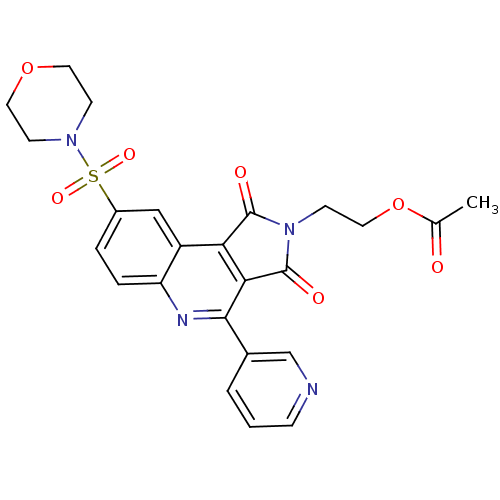 (2-[12-(morpholine-4-sulfonyl)-3,5-dioxo-7-(pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10373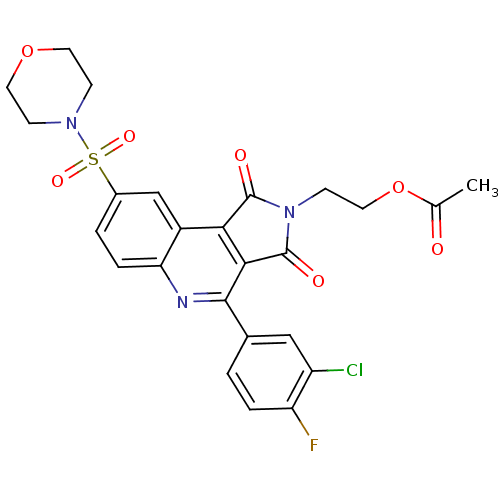 (2-[7-(3-chloro-4-fluorophenyl)-12-(morpholine-4-su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10361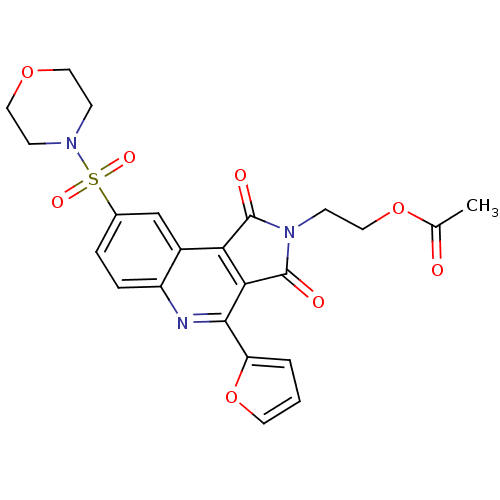 (2-[7-(furan-2-yl)-12-(morpholine-4-sulfonyl)-3,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10359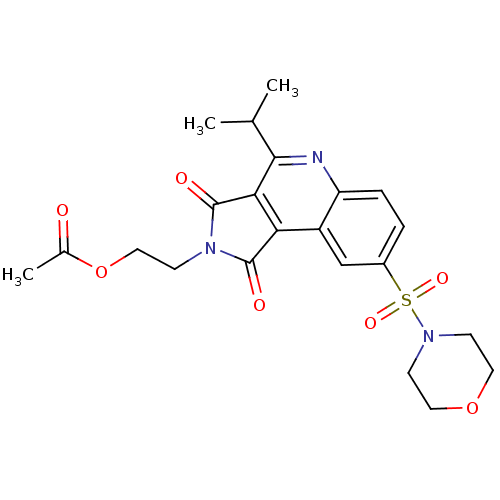 (2-[12-(morpholine-4-sulfonyl)-3,5-dioxo-7-(propan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10372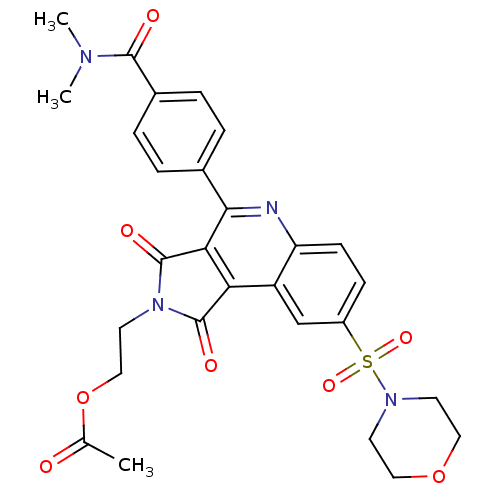 (2-{7-[4-(dimethylcarbamoyl)phenyl]-12-(morpholine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10365 (pyrrolo[3,4-c]-quinoline-1,3-dione 21a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 255 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10364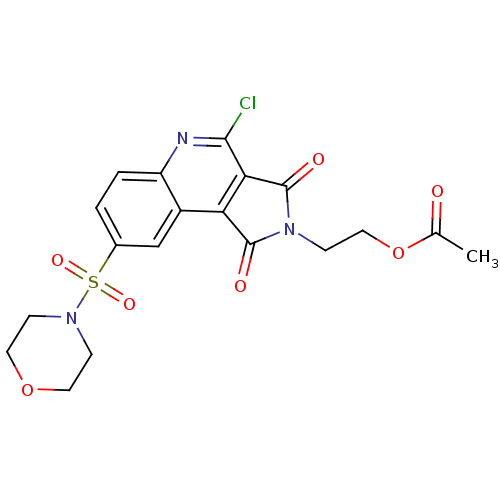 (2-[7-chloro-12-(morpholine-4-sulfonyl)-3,5-dioxo-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 294 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50313079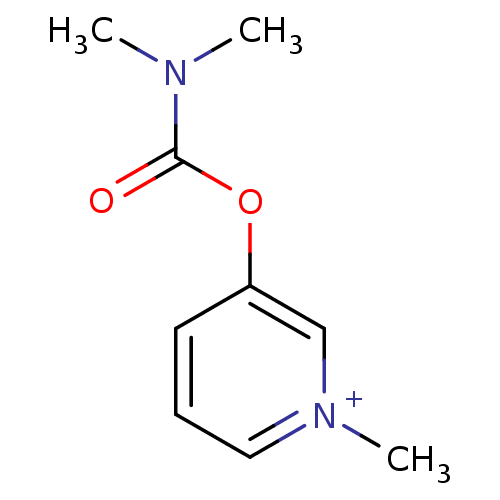 (3-Dimethylcarbamoyloxy-1-methyl-pyridinium; bromid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10370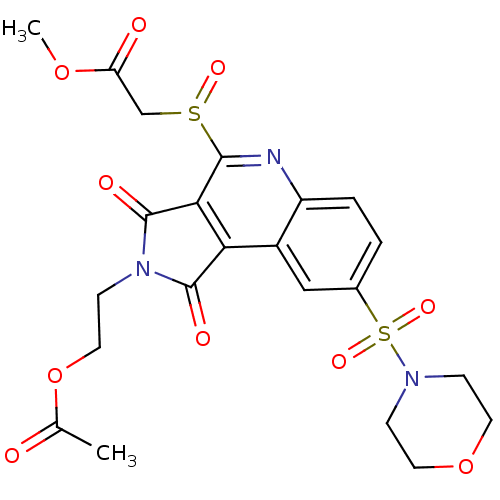 (methyl 2-({4-[2-(acetyloxy)ethyl]-12-(morpholine-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 352 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10371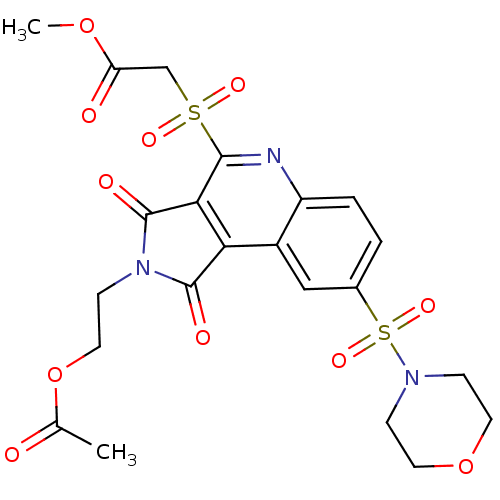 (methyl 2-({4-[2-(acetyloxy)ethyl]-12-(morpholine-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 362 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189893 (CHEMBL3828679) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189893 (CHEMBL3828679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50313079 (3-Dimethylcarbamoyloxy-1-methyl-pyridinium; bromid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10366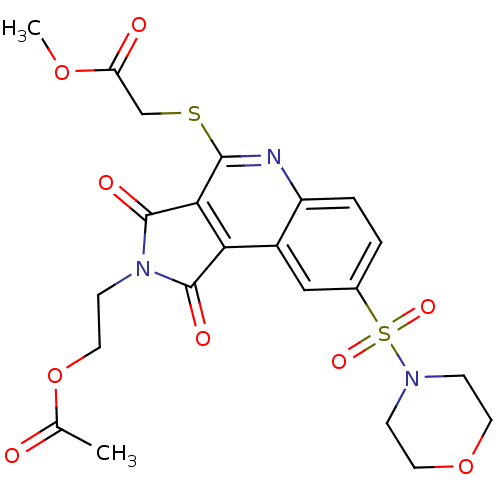 (methyl 2-({4-[2-(acetyloxy)ethyl]-12-(morpholine-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189894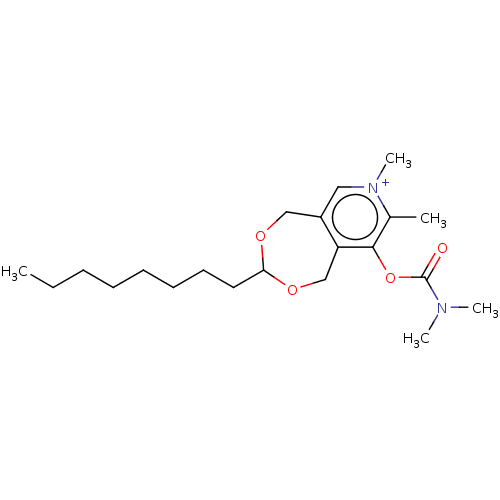 (CHEMBL3827011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189894 (CHEMBL3827011) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189895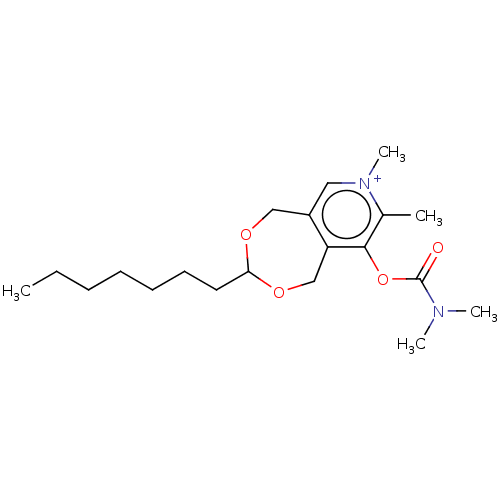 (CHEMBL3828028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10368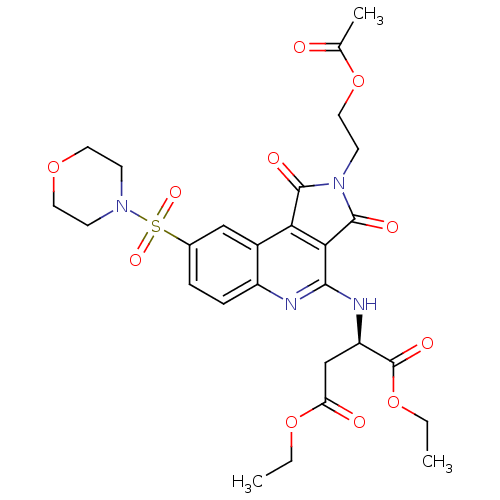 (1,4-diethyl (2R)-2-({4-[2-(acetyloxy)ethyl]-12-(mo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.19E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189895 (CHEMBL3828028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10363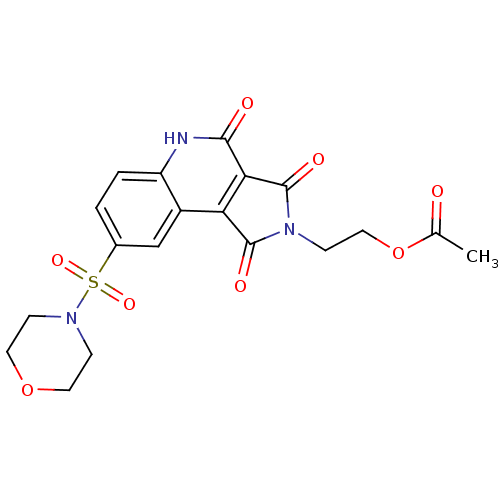 (2-[7-hydroxy-12-(morpholine-4-sulfonyl)-3,5-dioxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10367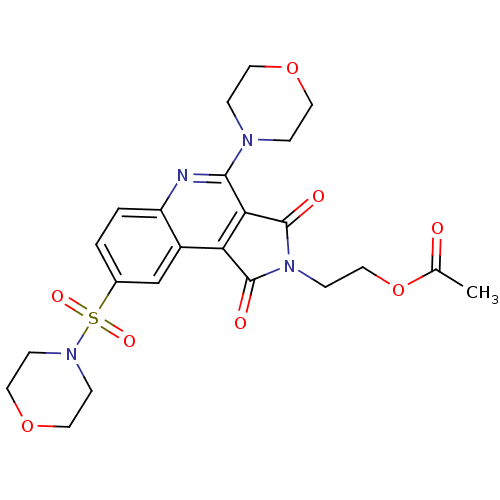 (2-[7-(morpholin-4-yl)-12-(morpholine-4-sulfonyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10369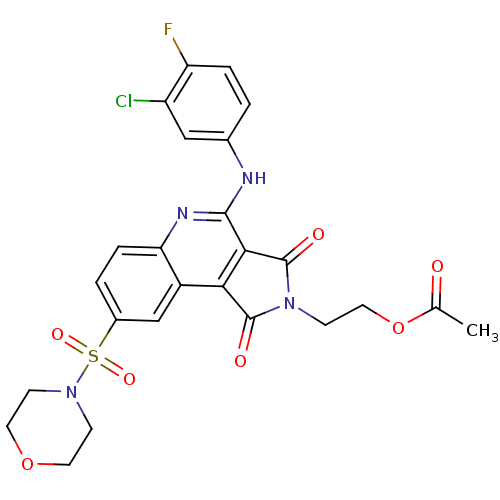 (2-{7-[(3-chloro-4-fluorophenyl)amino]-12-(morpholi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Chemical Diversity Research Institute | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 3680-3 (2005) Article DOI: 10.1021/jm048987t BindingDB Entry DOI: 10.7270/Q2QC01QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189896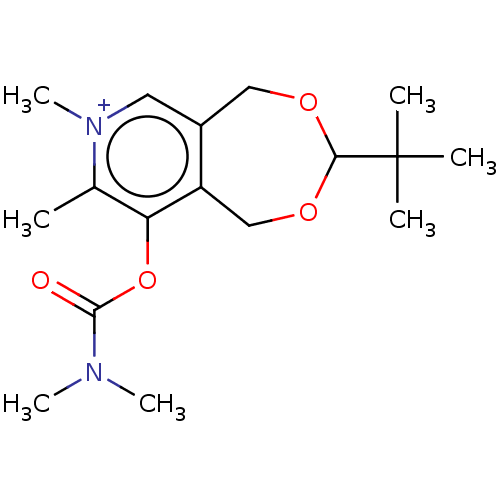 (CHEMBL3827624) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189892 (CHEMBL3827856) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189888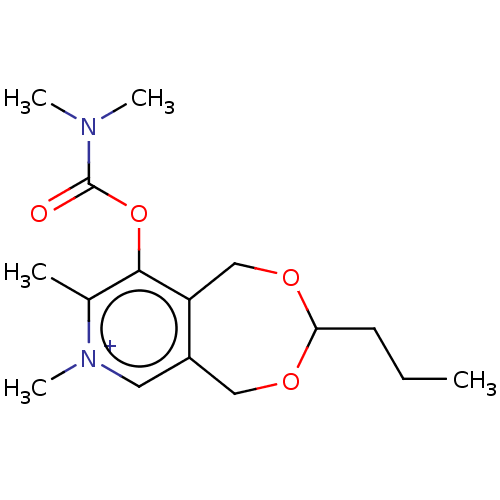 (CHEMBL3827449) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189891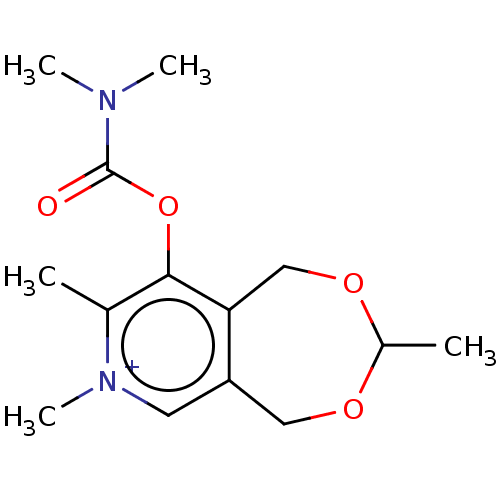 (CHEMBL3828644) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189887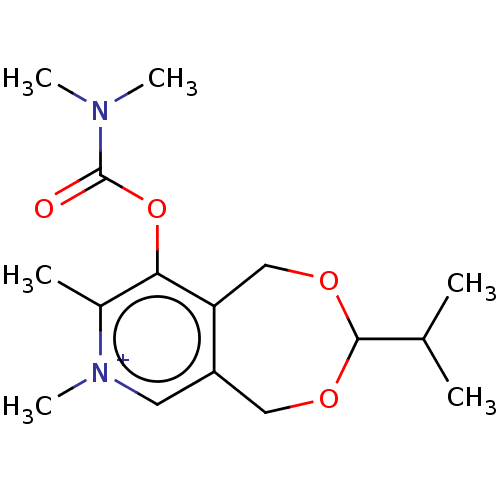 (CHEMBL3827347) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189889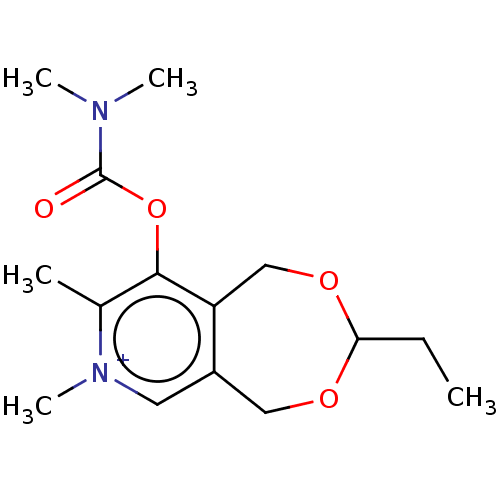 (CHEMBL3828273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189896 (CHEMBL3827624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189888 (CHEMBL3827449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189892 (CHEMBL3827856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50189890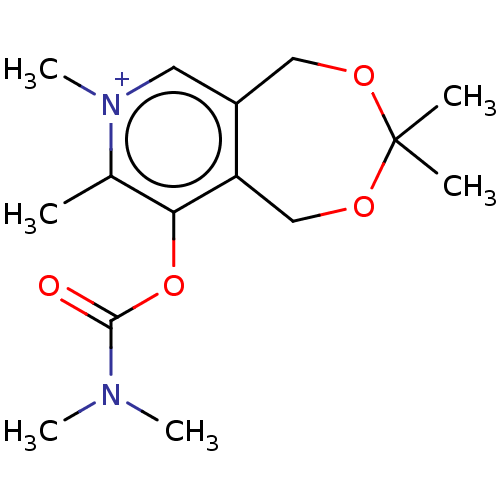 (CHEMBL3828739) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human AChE assessed as acetylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2 mins by... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189889 (CHEMBL3828273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189887 (CHEMBL3827347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189891 (CHEMBL3828644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50189890 (CHEMBL3828739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kazan (Volga region) Federal University Curated by ChEMBL | Assay Description Inhibition of human plasma BChE assessed as butyrylthiocholine hydrolysis preincubated for 10 mins followed by addition of substrate measured after 2... | Bioorg Med Chem Lett 26: 4092-4 (2016) Article DOI: 10.1016/j.bmcl.2016.06.070 BindingDB Entry DOI: 10.7270/Q2BR8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||