 Found 128 hits with Last Name = 'ballard' and Initial = 'te'
Found 128 hits with Last Name = 'ballard' and Initial = 'te' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450885
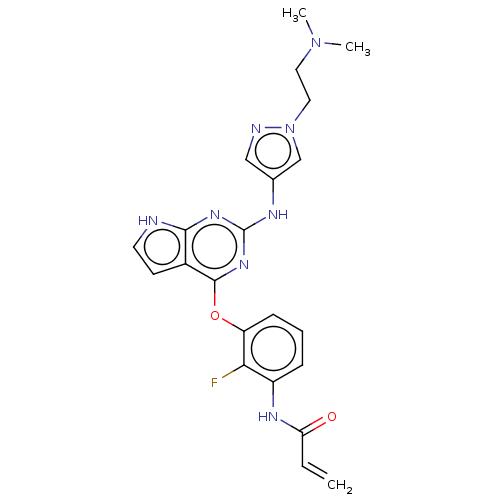
(CHEMBL4216749)Show SMILES CN(C)CCn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3F)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C22H23FN8O2/c1-4-18(32)27-16-6-5-7-17(19(16)23)33-21-15-8-9-24-20(15)28-22(29-21)26-14-12-25-31(13-14)11-10-30(2)3/h4-9,12-13H,1,10-11H2,2-3H3,(H,27,32)(H2,24,26,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50112173
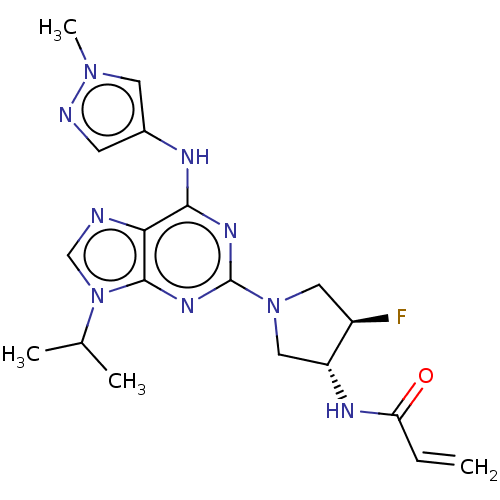
(CHEMBL3608429)Show SMILES CC(C)n1cnc2c(Nc3cnn(C)c3)nc(nc12)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C19H24FN9O/c1-5-15(30)24-14-9-28(8-13(14)20)19-25-17(23-12-6-22-27(4)7-12)16-18(26-19)29(10-21-16)11(2)3/h5-7,10-11,13-14H,1,8-9H2,2-4H3,(H,24,30)(H,23,25,26)/t13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450887
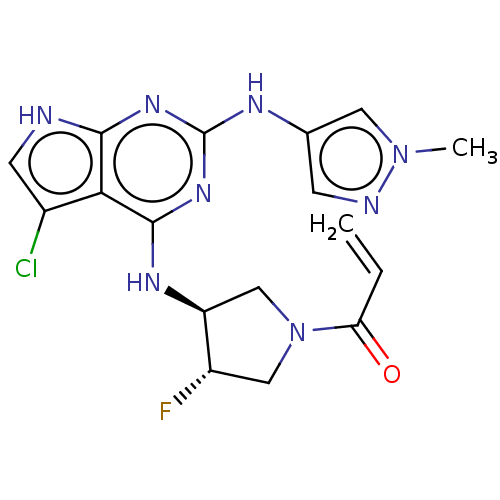
(CHEMBL4211367)Show SMILES Cn1cc(Nc2nc(N[C@H]3CN(C[C@@H]3F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C17H18ClFN8O/c1-3-13(28)27-7-11(19)12(8-27)23-16-14-10(18)5-20-15(14)24-17(25-16)22-9-4-21-26(2)6-9/h3-6,11-12H,1,7-8H2,2H3,(H3,20,22,23,24,25)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450884
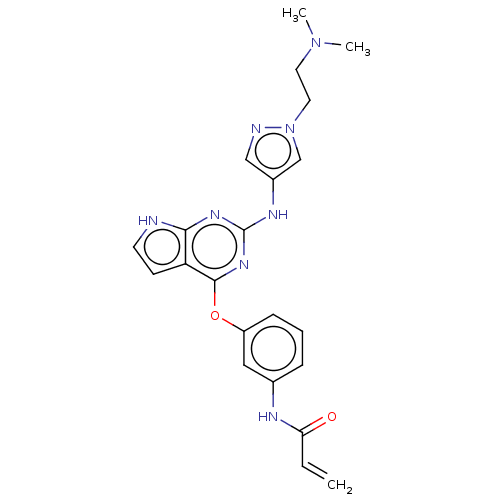
(CHEMBL4211782)Show SMILES CN(C)CCn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C22H24N8O2/c1-4-19(31)25-15-6-5-7-17(12-15)32-21-18-8-9-23-20(18)27-22(28-21)26-16-13-24-30(14-16)11-10-29(2)3/h4-9,12-14H,1,10-11H2,2-3H3,(H,25,31)(H2,23,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450886
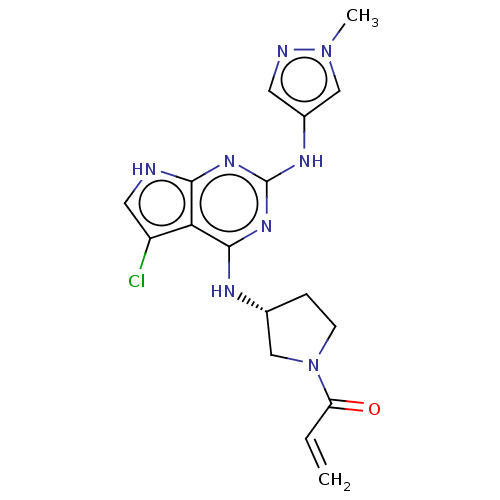
(CHEMBL4206481)Show SMILES Cn1cc(Nc2nc(N[C@@H]3CCN(C3)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C17H19ClN8O/c1-3-13(27)26-5-4-10(9-26)21-16-14-12(18)7-19-15(14)23-17(24-16)22-11-6-20-25(2)8-11/h3,6-8,10H,1,4-5,9H2,2H3,(H3,19,21,22,23,24)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450871

(CHEMBL4205392)Show SMILES CCn1cnc2c(Nc3cn(C)nc3OC)nc(nc12)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C19H24FN9O2/c1-5-14(30)22-12-9-29(7-11(12)20)19-24-16(15-17(25-19)28(6-2)10-21-15)23-13-8-27(3)26-18(13)31-4/h5,8,10-12H,1,6-7,9H2,2-4H3,(H,22,30)(H,23,24,25)/t11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450868
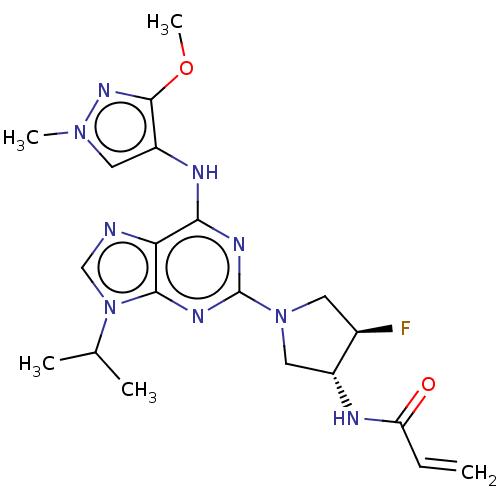
(CHEMBL4216679)Show SMILES COc1nn(C)cc1Nc1nc(nc2n(cnc12)C(C)C)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C20H26FN9O2/c1-6-15(31)23-13-9-29(7-12(13)21)20-25-17(24-14-8-28(4)27-19(14)32-5)16-18(26-20)30(10-22-16)11(2)3/h6,8,10-13H,1,7,9H2,2-5H3,(H,23,31)(H,24,25,26)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450875
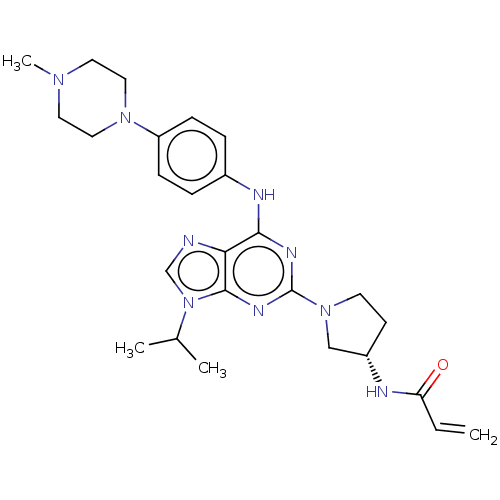
(CHEMBL4208811)Show SMILES CC(C)n1cnc2c(Nc3ccc(cc3)N3CCN(C)CC3)nc(nc12)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C26H35N9O/c1-5-22(36)28-20-10-11-34(16-20)26-30-24(23-25(31-26)35(17-27-23)18(2)3)29-19-6-8-21(9-7-19)33-14-12-32(4)13-15-33/h5-9,17-18,20H,1,10-16H2,2-4H3,(H,28,36)(H,29,30,31)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450870
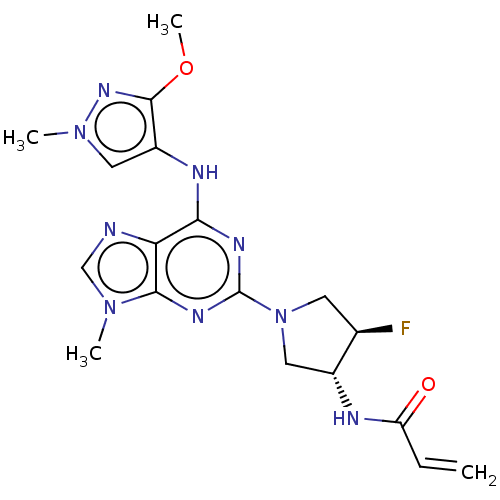
(Mavelertinib | PF-06747775)Show SMILES COc1nn(C)cc1Nc1nc(nc2n(C)cnc12)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C18H22FN9O2/c1-5-13(29)21-11-8-28(6-10(11)19)18-23-15(14-16(24-18)26(2)9-20-14)22-12-7-27(3)25-17(12)30-4/h5,7,9-11H,1,6,8H2,2-4H3,(H,21,29)(H,22,23,24)/t10-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50112176

(CHEMBL3608432)Show SMILES CC(C)n1cnc2c(Nc3cn(C)nc3C)nc(nc12)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C20H26FN9O/c1-6-16(31)23-15-9-29(7-13(15)21)20-25-18(24-14-8-28(5)27-12(14)4)17-19(26-20)30(10-22-17)11(2)3/h6,8,10-11,13,15H,1,7,9H2,2-5H3,(H,23,31)(H,24,25,26)/t13-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450866
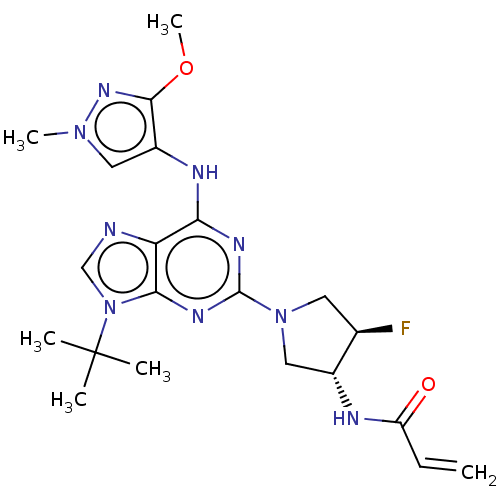
(CHEMBL4208829)Show SMILES COc1nn(C)cc1Nc1nc(nc2n(cnc12)C(C)(C)C)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C21H28FN9O2/c1-7-15(32)24-13-10-30(8-12(13)22)20-26-17(25-14-9-29(5)28-19(14)33-6)16-18(27-20)31(11-23-16)21(2,3)4/h7,9,11-13H,1,8,10H2,2-6H3,(H,24,32)(H,25,26,27)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450869
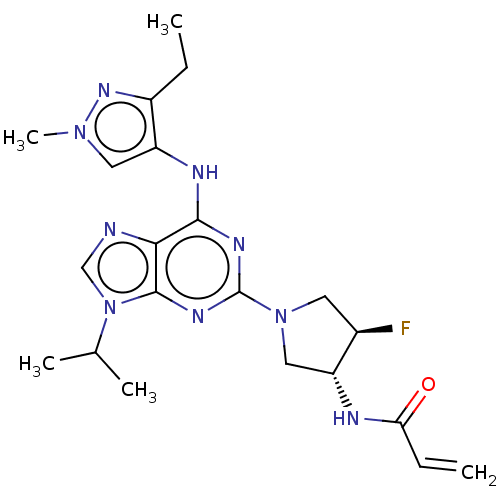
(CHEMBL4218154)Show SMILES CCc1nn(C)cc1Nc1nc(nc2n(cnc12)C(C)C)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C21H28FN9O/c1-6-14-16(9-29(5)28-14)25-19-18-20(31(11-23-18)12(3)4)27-21(26-19)30-8-13(22)15(10-30)24-17(32)7-2/h7,9,11-13,15H,2,6,8,10H2,1,3-5H3,(H,24,32)(H,25,26,27)/t13-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450873
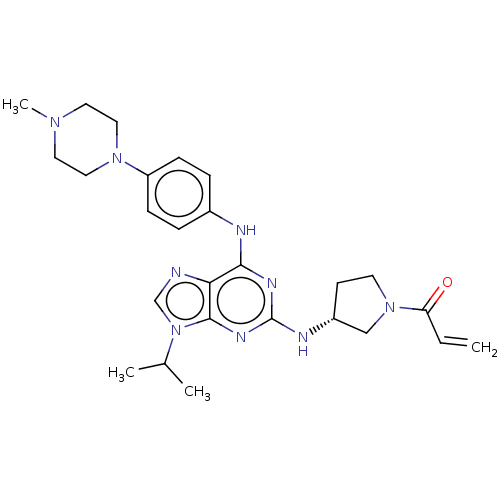
(CHEMBL4206312)Show SMILES CC(C)n1cnc2c(Nc3ccc(cc3)N3CCN(C)CC3)nc(N[C@@H]3CCN(C3)C(=O)C=C)nc12 |r| Show InChI InChI=1S/C26H35N9O/c1-5-22(36)34-11-10-20(16-34)29-26-30-24(23-25(31-26)35(17-27-23)18(2)3)28-19-6-8-21(9-7-19)33-14-12-32(4)13-15-33/h5-9,17-18,20H,1,10-16H2,2-4H3,(H2,28,29,30,31)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450865
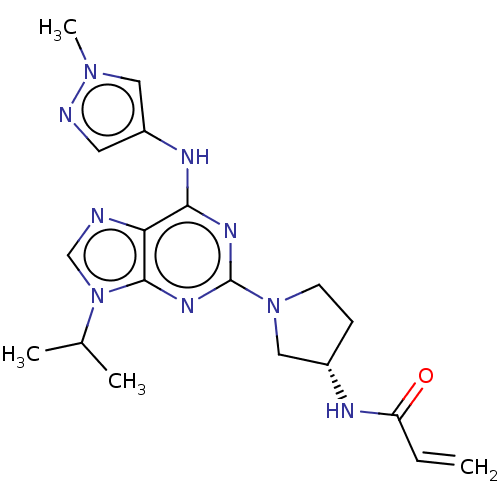
(CHEMBL4212326)Show SMILES CC(C)n1cnc2c(Nc3cnn(C)c3)nc(nc12)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C19H25N9O/c1-5-15(29)22-13-6-7-27(10-13)19-24-17(23-14-8-21-26(4)9-14)16-18(25-19)28(11-20-16)12(2)3/h5,8-9,11-13H,1,6-7,10H2,2-4H3,(H,22,29)(H,23,24,25)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450889
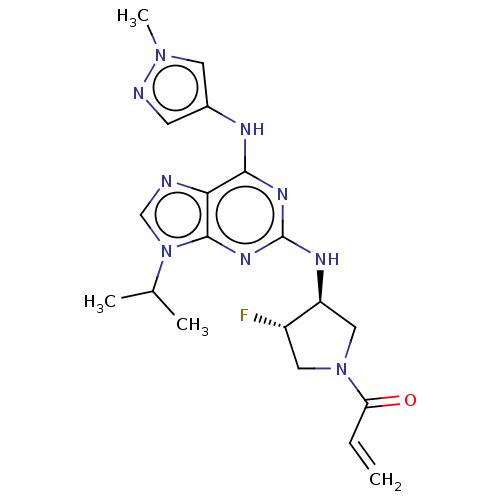
(CHEMBL4204498)Show SMILES CC(C)n1cnc2c(Nc3cnn(C)c3)nc(N[C@H]3CN(C[C@@H]3F)C(=O)C=C)nc12 |r| Show InChI InChI=1S/C19H24FN9O/c1-5-15(30)28-8-13(20)14(9-28)24-19-25-17(23-12-6-22-27(4)7-12)16-18(26-19)29(10-21-16)11(2)3/h5-7,10-11,13-14H,1,8-9H2,2-4H3,(H2,23,24,25,26)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450879
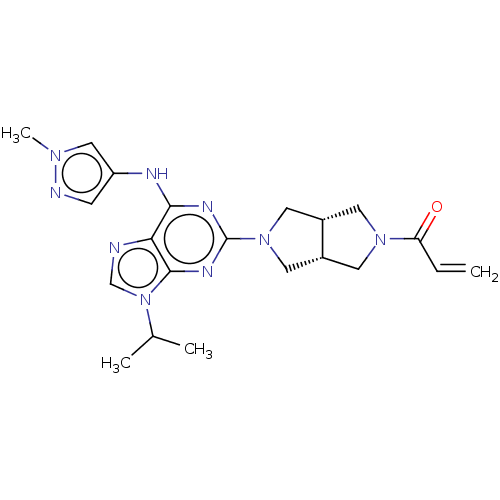
(CHEMBL4207951)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(Nc2cnn(C)c2)c2ncn(C(C)C)c2n1)C(=O)C=C |r| Show InChI InChI=1S/C21H27N9O/c1-5-17(31)28-7-14-9-29(10-15(14)8-28)21-25-19(24-16-6-23-27(4)11-16)18-20(26-21)30(12-22-18)13(2)3/h5-6,11-15H,1,7-10H2,2-4H3,(H,24,25,26)/t14-,15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4-mediated N-demethylation in human liver microsomes |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450888
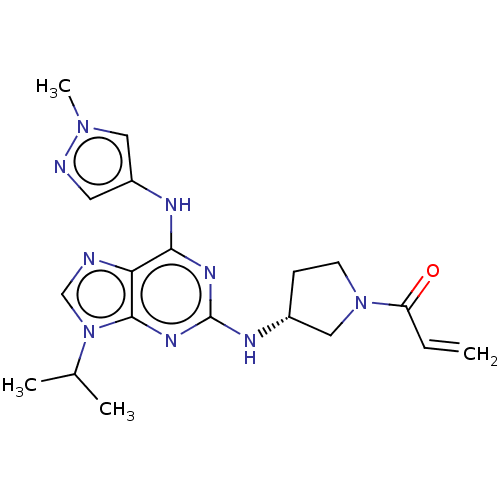
(CHEMBL4209368)Show SMILES CC(C)n1cnc2c(Nc3cnn(C)c3)nc(N[C@@H]3CCN(C3)C(=O)C=C)nc12 |r| Show InChI InChI=1S/C19H25N9O/c1-5-15(29)27-7-6-13(10-27)23-19-24-17(22-14-8-21-26(4)9-14)16-18(25-19)28(11-20-16)12(2)3/h5,8-9,11-13H,1,6-7,10H2,2-4H3,(H2,22,23,24,25)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450880
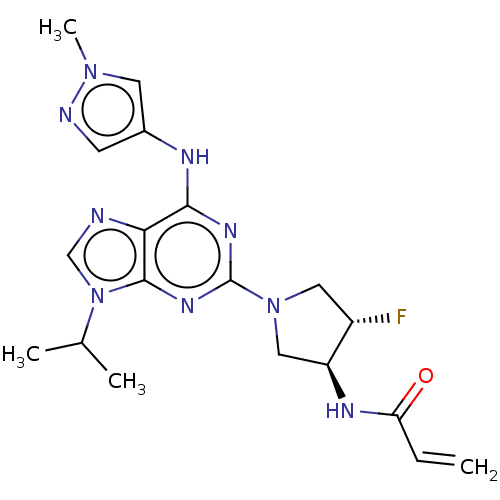
(CHEMBL4204566)Show SMILES CC(C)n1cnc2c(Nc3cnn(C)c3)nc(nc12)N1C[C@H](F)[C@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C19H24FN9O/c1-5-15(30)24-14-9-28(8-13(14)20)19-25-17(23-12-6-22-27(4)7-12)16-18(26-19)29(10-21-16)11(2)3/h5-7,10-11,13-14H,1,8-9H2,2-4H3,(H,24,30)(H,23,25,26)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450882
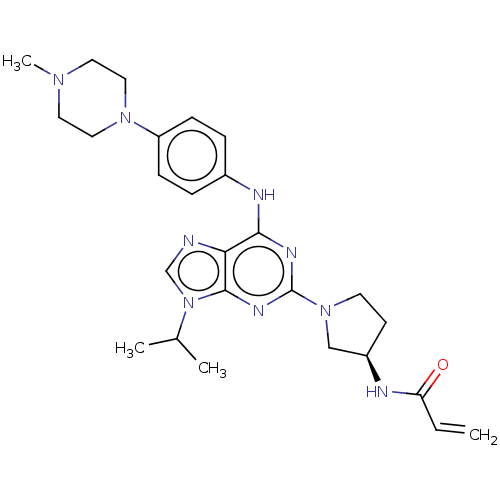
(CHEMBL4207425)Show SMILES CC(C)n1cnc2c(Nc3ccc(cc3)N3CCN(C)CC3)nc(nc12)N1CC[C@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C26H35N9O/c1-5-22(36)28-20-10-11-34(16-20)26-30-24(23-25(31-26)35(17-27-23)18(2)3)29-19-6-8-21(9-7-19)33-14-12-32(4)13-15-33/h5-9,17-18,20H,1,10-16H2,2-4H3,(H,28,36)(H,29,30,31)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450890
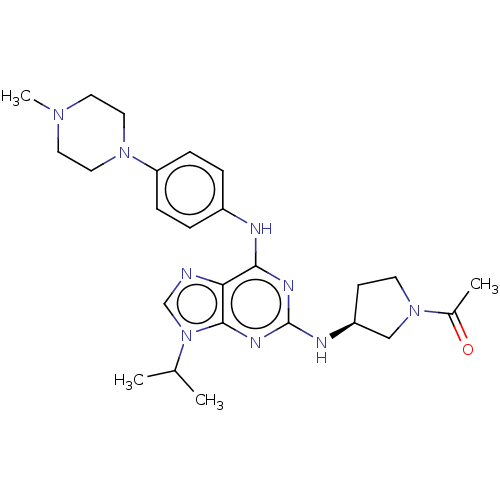
(CHEMBL4206604)Show SMILES CC(C)n1cnc2c(Nc3ccc(cc3)N3CCN(C)CC3)nc(N[C@H]3CCN(C3)C(C)=O)nc12 |r| Show InChI InChI=1S/C25H35N9O/c1-17(2)34-16-26-22-23(27-19-5-7-21(8-6-19)32-13-11-31(4)12-14-32)29-25(30-24(22)34)28-20-9-10-33(15-20)18(3)35/h5-8,16-17,20H,9-15H2,1-4H3,(H2,27,28,29,30)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450874
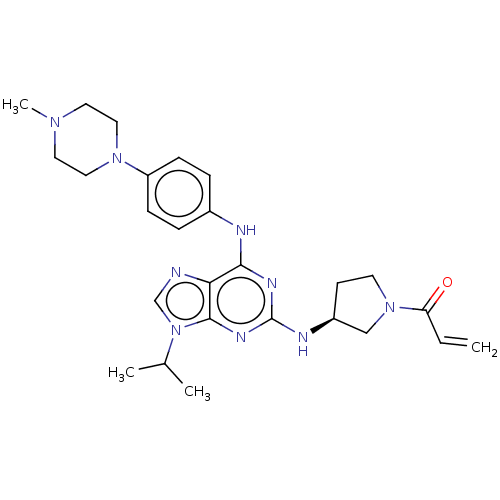
(CHEMBL4213741)Show SMILES CC(C)n1cnc2c(Nc3ccc(cc3)N3CCN(C)CC3)nc(N[C@H]3CCN(C3)C(=O)C=C)nc12 |r| Show InChI InChI=1S/C26H35N9O/c1-5-22(36)34-11-10-20(16-34)29-26-30-24(23-25(31-26)35(17-27-23)18(2)3)28-19-6-8-21(9-7-19)33-14-12-32(4)13-15-33/h5-9,17-18,20H,1,10-16H2,2-4H3,(H2,28,29,30,31)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4-mediated testosterone-6-beta hydroxylation in human liver microsome |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450883
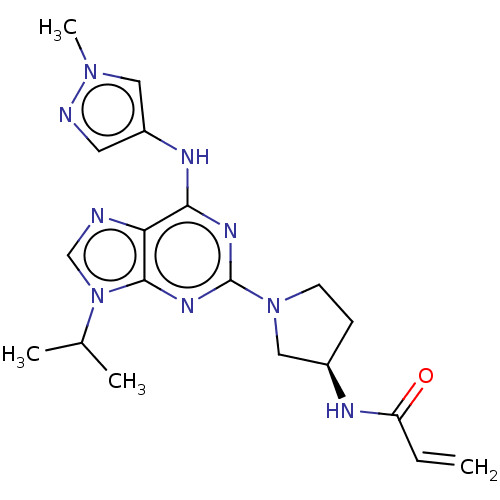
(CHEMBL4203072)Show SMILES CC(C)n1cnc2c(Nc3cnn(C)c3)nc(nc12)N1CC[C@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C19H25N9O/c1-5-15(29)22-13-6-7-27(10-13)19-24-17(23-14-8-21-26(4)9-14)16-18(25-19)28(11-20-16)12(2)3/h5,8-9,11-13H,1,6-7,10H2,2-4H3,(H,22,29)(H,23,24,25)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4-mediated testosterone-6-beta hydroxylation in human liver microsome |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50397662
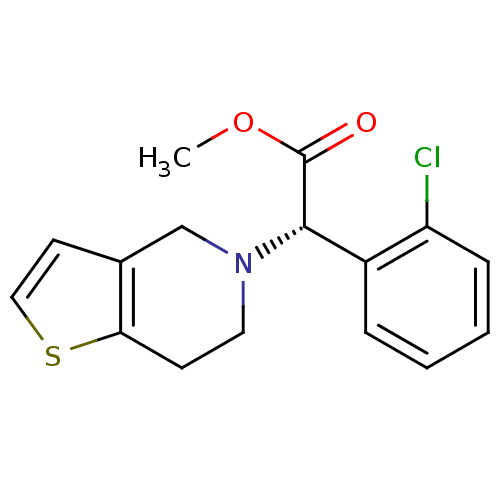
(CLOPIDOGREL)Show InChI InChI=1S/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2B6 in human liver microsomes |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4-mediated testosterone-6-beta hydroxylation in human liver microsome |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4-mediated testosterone-6-beta hydroxylation in human liver microsome |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50366785

(NELFINAVIR)Show SMILES Cc1c(O)cccc1C(=O)N[C@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26+,27-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4-mediated testosterone-6-beta hydroxylation in human liver microsome |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50069447
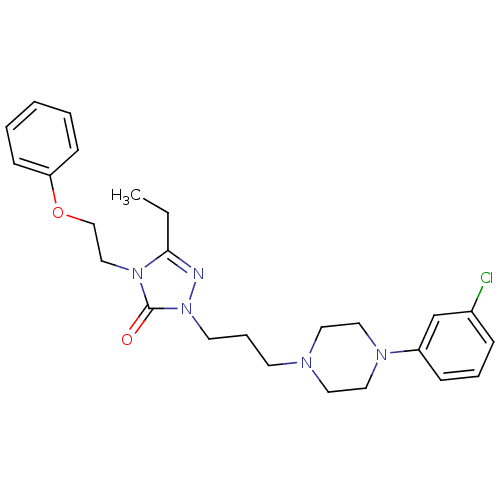
(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes assessed as quasi-irreversible complex formation |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50117922
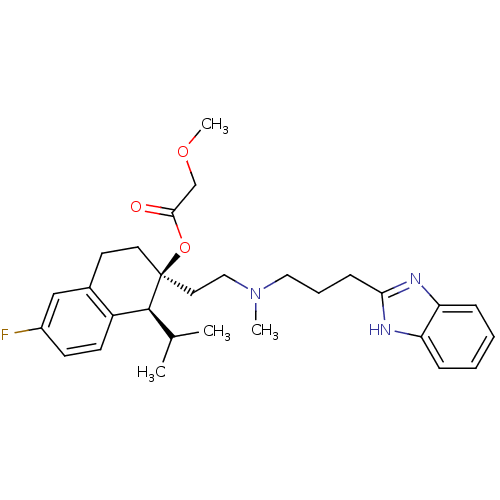
((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...)Show SMILES COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C |r| Show InChI InChI=1S/C29H38FN3O3/c1-20(2)28-23-12-11-22(30)18-21(23)13-14-29(28,36-27(34)19-35-4)15-17-33(3)16-7-10-26-31-24-8-5-6-9-25(24)32-26/h5-6,8-9,11-12,18,20,28H,7,10,13-17,19H2,1-4H3,(H,31,32)/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM85509
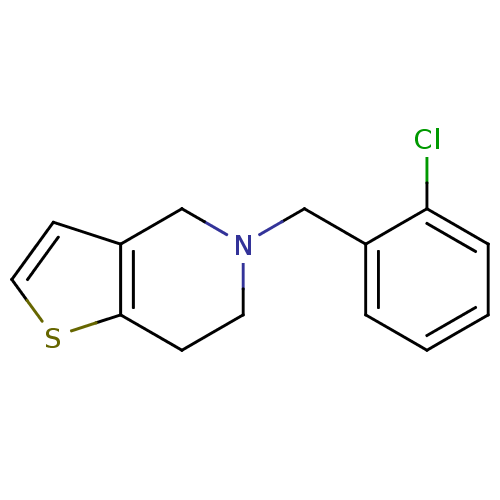
(CAS_55142-85-3 | NSC_5472 | Ticlopidine)Show InChI InChI=1S/C14H14ClNS/c15-13-4-2-1-3-11(13)9-16-7-5-14-12(10-16)6-8-17-14/h1-4,6,8H,5,7,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2C19 |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318884

(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50397662

(CLOPIDOGREL)Show InChI InChI=1S/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2C19 |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4-mediated midazolam 1'-hydroxylation |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50397664
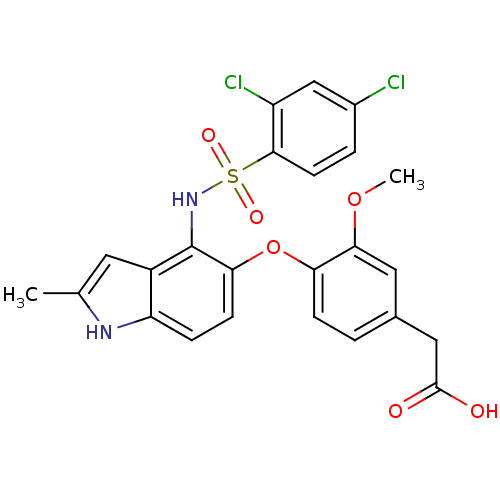
(CHEMBL2181816)Show SMILES COc1cc(CC(O)=O)ccc1Oc1ccc2[nH]c(C)cc2c1NS(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H20Cl2N2O6S/c1-13-9-16-18(27-13)5-7-20(34-19-6-3-14(11-23(29)30)10-21(19)33-2)24(16)28-35(31,32)22-8-4-15(25)12-17(22)26/h3-10,12,27-28H,11H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes in presence of NADPH |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50397663
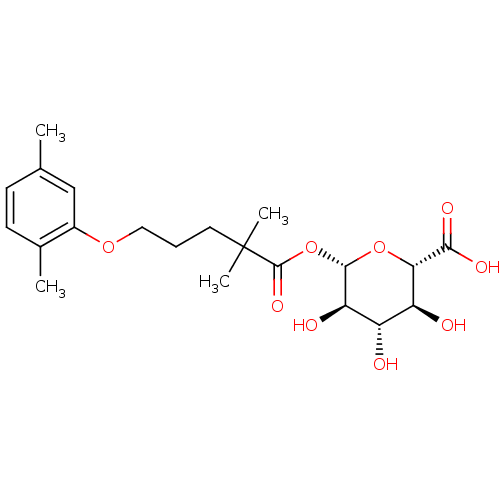
(CHEMBL2181817)Show SMILES Cc1ccc(C)c(OCCCC(C)(C)C(=O)O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1 |r| Show InChI InChI=1S/C21H30O9/c1-11-6-7-12(2)13(10-11)28-9-5-8-21(3,4)20(27)30-19-16(24)14(22)15(23)17(29-19)18(25)26/h6-7,10,14-17,19,22-24H,5,8-9H2,1-4H3,(H,25,26)/t14-,15-,16+,17-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2C8 in human liver microsomes |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50168724

(1-[(R)-2-[((1S,2S)-1-Amino-1,2,3,4-tetrahydro-naph...)Show SMILES CC(C)(C)NC(=O)C1(CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1CCc2ccccc2[C@H]1N)C1CCCCC1 Show InChI InChI=1S/C36H49ClN4O3/c1-35(2,3)40-34(44)36(26-10-5-4-6-11-26)19-21-41(22-20-36)33(43)30(23-24-13-16-27(37)17-14-24)39-32(42)29-18-15-25-9-7-8-12-28(25)31(29)38/h7-9,12-14,16-17,26,29-31H,4-6,10-11,15,18-23,38H2,1-3H3,(H,39,42)(H,40,44)/t29-,30+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496623

(CHEMBL3134498)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:38| Show InChI InChI=1S/C42H53N5O6/c1-27(38(49)47-37-40(51)44-33-23-15-14-22-32(33)36(46-37)30-20-12-7-13-21-30)43-39(50)31(24-28-16-8-5-9-17-28)26-35(48)34(25-29-18-10-6-11-19-29)45-41(52)53-42(2,3)4/h5-6,8-11,14-19,22-23,27,30-31,34-35,37,48H,7,12-13,20-21,24-26H2,1-4H3,(H,43,50)(H,44,51)(H,45,52)(H,47,49)/t27-,31+,34-,35+,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using Notch1 substrate assessed as Notch1-NICD production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496623

(CHEMBL3134498)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:38| Show InChI InChI=1S/C42H53N5O6/c1-27(38(49)47-37-40(51)44-33-23-15-14-22-32(33)36(46-37)30-20-12-7-13-21-30)43-39(50)31(24-28-16-8-5-9-17-28)26-35(48)34(25-29-18-10-6-11-19-29)45-41(52)53-42(2,3)4/h5-6,8-11,14-19,22-23,27,30-31,34-35,37,48H,7,12-13,20-21,24-26H2,1-4H3,(H,43,50)(H,44,51)(H,45,52)(H,47,49)/t27-,31+,34-,35+,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using APP substrate assessed as amyloid-beta40 production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496624

(CHEMBL3134497)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(=O)c1ccc(OCC#C)cc1)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:53| Show InChI InChI=1S/C54H55N5O7/c1-3-31-66-43-29-27-40(28-30-43)49(61)39-23-25-41(26-24-39)52(63)57-46(33-37-17-9-5-10-18-37)47(60)34-42(32-36-15-7-4-8-16-36)53(64)55-35(2)51(62)59-50-54(65)56-45-22-14-13-21-44(45)48(58-50)38-19-11-6-12-20-38/h1,4-5,7-10,13-18,21-30,35,38,42,46-47,50,60H,6,11-12,19-20,31-34H2,2H3,(H,55,64)(H,56,65)(H,57,63)(H,59,62)/t35-,42+,46-,47+,50-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using APP substrate assessed as amyloid-beta40 production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50210162
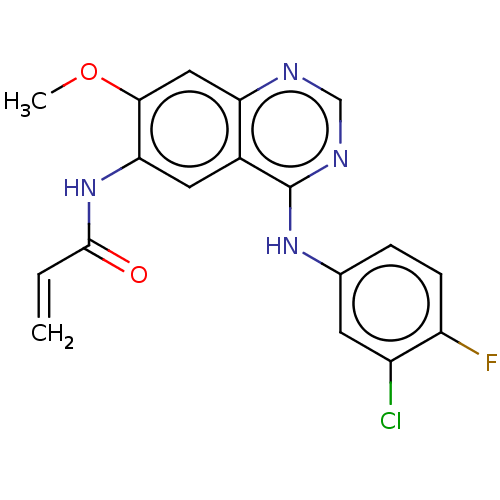
(CHEMBL3883534)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)C=C Show InChI InChI=1S/C18H14ClFN4O2/c1-3-17(25)24-15-7-11-14(8-16(15)26-2)21-9-22-18(11)23-10-4-5-13(20)12(19)6-10/h3-9H,1H2,2H3,(H,24,25)(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion mutant phosphorylation in human PC9 cells preincubated for 2 hrs followed by EGF stimulation for 10 mins by sandw... |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450868

(CHEMBL4216679)Show SMILES COc1nn(C)cc1Nc1nc(nc2n(cnc12)C(C)C)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C20H26FN9O2/c1-6-15(31)23-13-9-29(7-12(13)21)20-25-17(24-14-8-28(4)27-19(14)32-5)16-18(26-20)30(10-22-16)11(2)3/h6,8,10-13H,1,7,9H2,2-5H3,(H,23,31)(H,24,25,26)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/exon 19 deletion mutant phosphorylation in human PC9-DRH cells preincubated for 2 hrs followed by EGF stimulation for 10 min... |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-3
(Homo sapiens (Human)) | BDBM50210162

(CHEMBL3883534)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)C=C Show InChI InChI=1S/C18H14ClFN4O2/c1-3-17(25)24-15-7-11-14(8-16(15)26-2)21-9-22-18(11)23-10-4-5-13(20)12(19)6-10/h3-9H,1H2,2H3,(H,24,25)(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant phosphorylation in human H3255 cells preincubated for 2 hrs followed by EGF stimulation for 10 mins by sandwich ELISA |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496624

(CHEMBL3134497)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(=O)c1ccc(OCC#C)cc1)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:53| Show InChI InChI=1S/C54H55N5O7/c1-3-31-66-43-29-27-40(28-30-43)49(61)39-23-25-41(26-24-39)52(63)57-46(33-37-17-9-5-10-18-37)47(60)34-42(32-36-15-7-4-8-16-36)53(64)55-35(2)51(62)59-50-54(65)56-45-22-14-13-21-44(45)48(58-50)38-19-11-6-12-20-38/h1,4-5,7-10,13-18,21-30,35,38,42,46-47,50,60H,6,11-12,19-20,31-34H2,2H3,(H,55,64)(H,56,65)(H,57,63)(H,59,62)/t35-,42+,46-,47+,50-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using Notch1 substrate assessed as Notch1-NICD production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450868

(CHEMBL4216679)Show SMILES COc1nn(C)cc1Nc1nc(nc2n(cnc12)C(C)C)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C20H26FN9O2/c1-6-15(31)23-13-9-29(7-12(13)21)20-25-17(24-14-8-28(4)27-19(14)32-5)16-18(26-20)30(10-22-16)11(2)3/h6,8,10-13H,1,7,9H2,2-5H3,(H,23,31)(H,24,25,26)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion mutant phosphorylation in human PC9 cells preincubated for 2 hrs followed by EGF stimulation for 10 mins by sandw... |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data