

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015471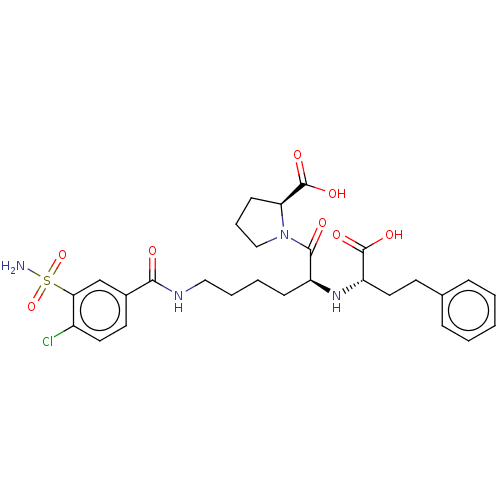 (1-[2-(1-Carboxy-3-phenyl-propylamino)-6-(4-chloro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015473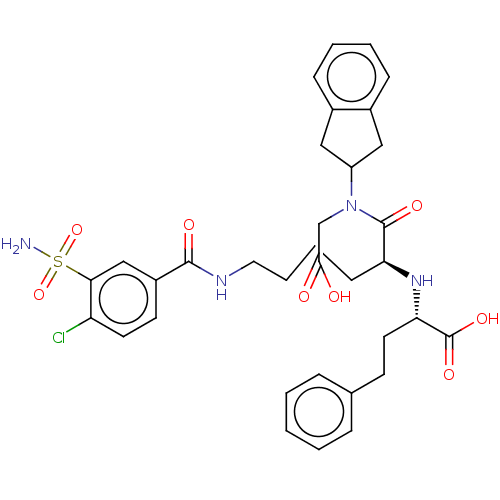 (2-[1-(Carboxymethyl-indan-2-yl-carbamoyl)-5-(4-chl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021340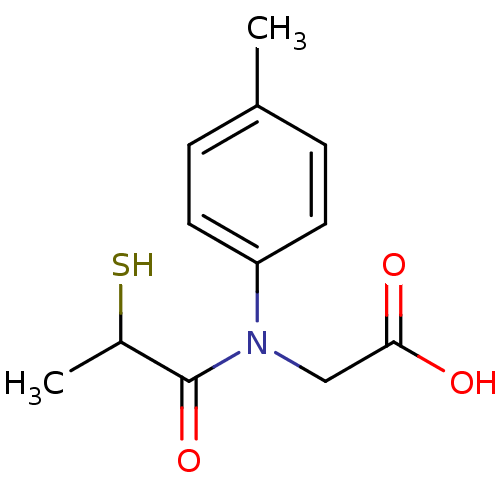 (CHEMBL166743 | [(2-Mercapto-propionyl)-p-tolyl-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015477 (2-[1-(Carboxymethyl-indan-2-yl-carbamoyl)-5-(4-chl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021332 (CHEMBL165952 | [Cyclopentyl-(2-mercapto-propionyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021338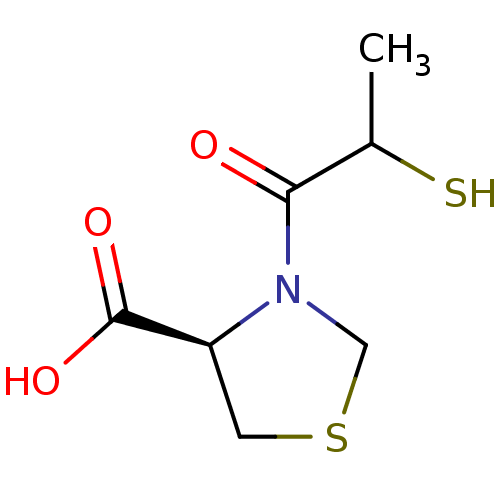 (3-(2-Mercapto-propionyl)-thiazolidine-4-carboxylic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of activity of rabbit lung Angiotensin I converting enzyme at pH 8.5; 0.14-10 | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021338 (3-(2-Mercapto-propionyl)-thiazolidine-4-carboxylic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50368115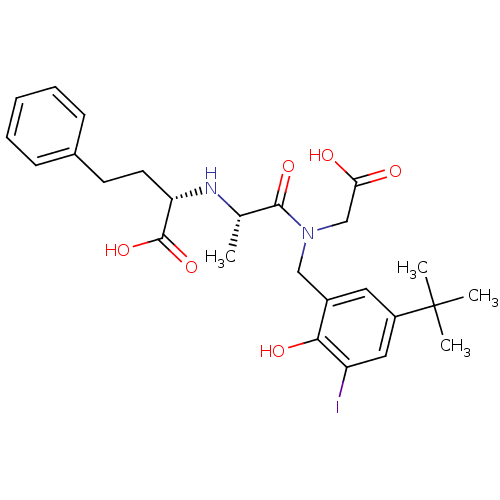 (CHEMBL1202592) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021336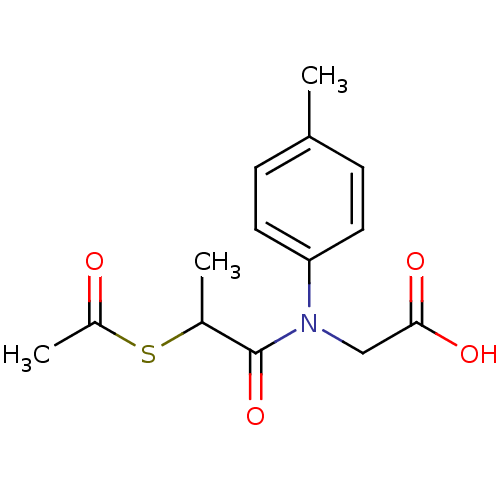 (CHEMBL355163 | [(2-Acetylsulfanyl-propionyl)-p-tol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015476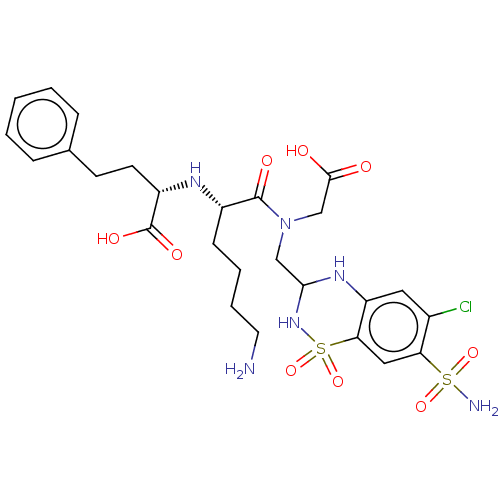 (2-{5-Amino-1-[carboxymethyl-(6-chloro-1,1-dioxo-7-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015483 (2-(1-{Carboxymethyl-[3-(6-chloro-1,1-dioxo-7-sulfa...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015484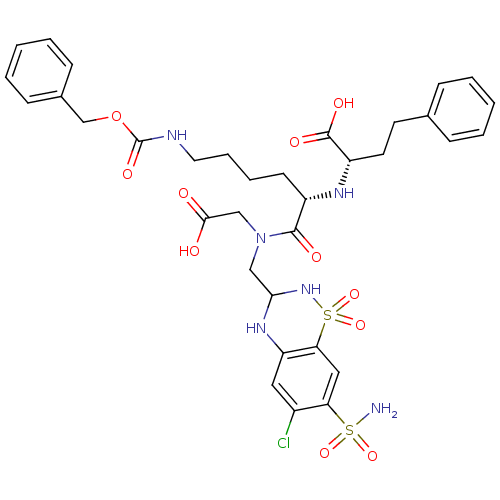 (2-{5-Benzyloxycarbonylamino-1-[carboxymethyl-(6-ch...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021333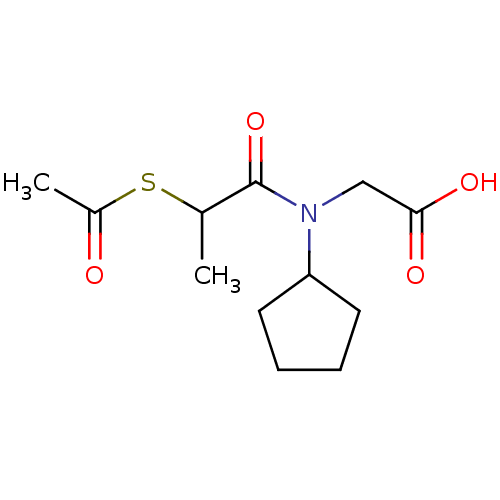 (CHEMBL167651 | [(2-Acetylsulfanyl-propionyl)-cyclo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015482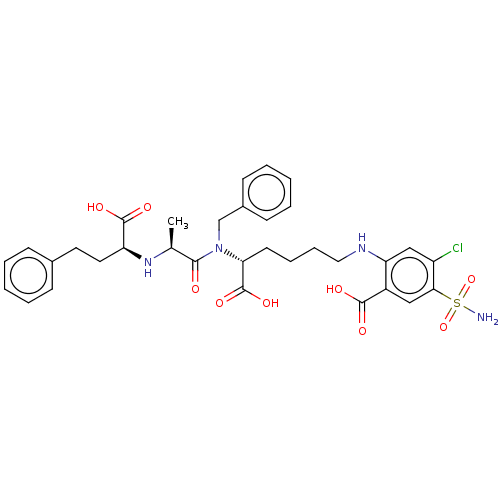 (2-(5-{Benzyl-[2-(1-carboxy-3-phenyl-propylamino)-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50421739 (CHEMBL2110192) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020805 ((2-Mercapto-propionylamino)-acetic acid | CHEMBL13...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015474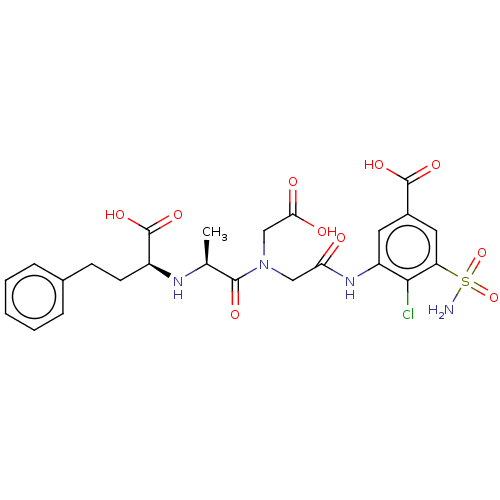 (3-(2-{Carboxymethyl-[2-(1-carboxy-3-phenyl-propyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015469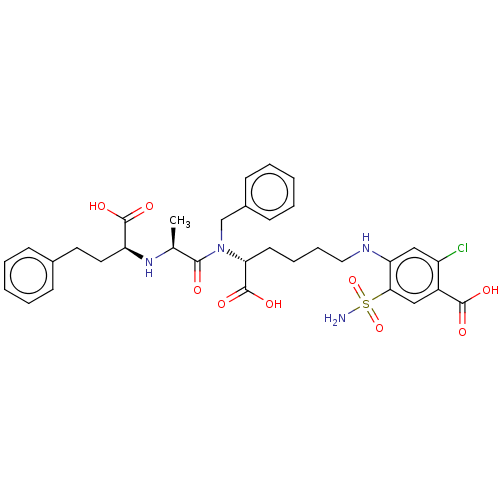 (4-(5-{Benzyl-[2-(1-carboxy-3-phenyl-propylamino)-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015467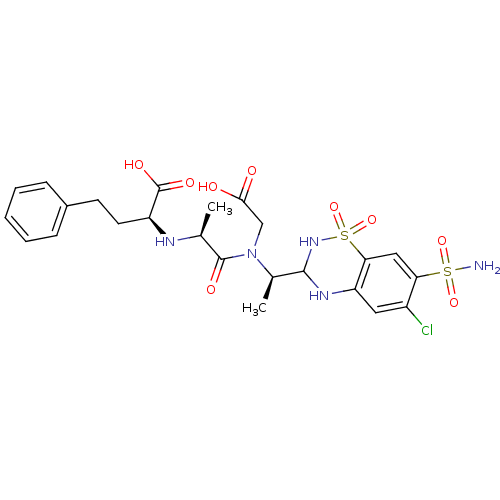 (2-(1-{Carboxymethyl-[1-(6-chloro-1,1-dioxo-7-sulfa...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015472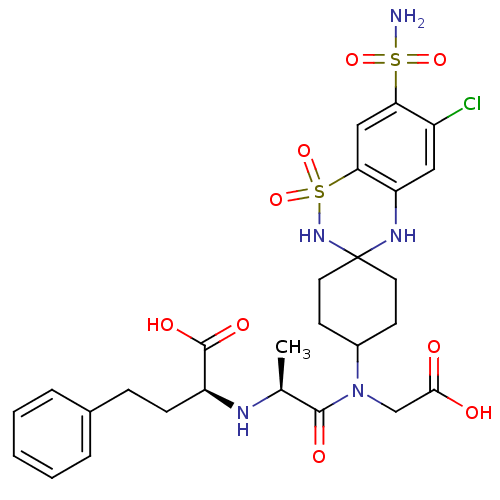 (CHEMBL41548 | N-[7-(Aminosulfonyl)-6-chlorospiro[2...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021343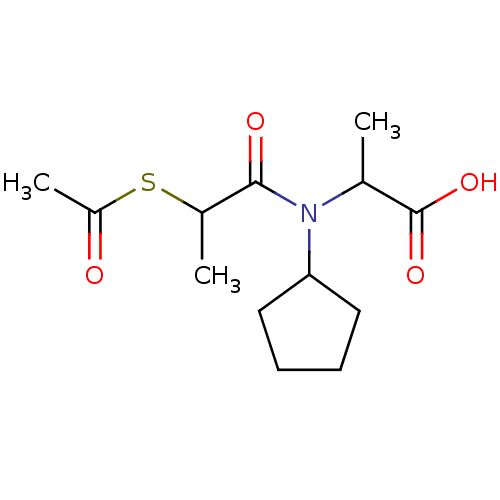 (2-[(2-Acetylsulfanyl-propionyl)-cyclopentyl-amino]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021339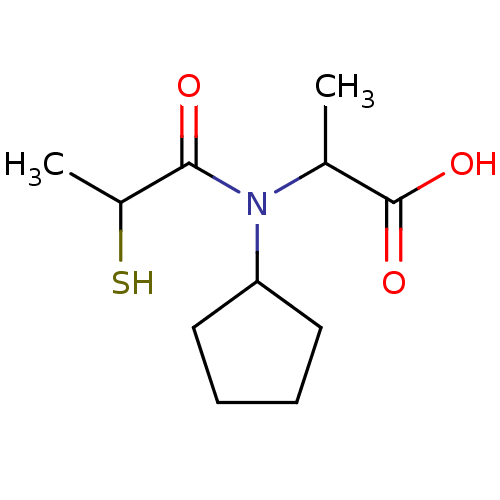 (2-[Cyclopentyl-(2-mercapto-propionyl)-amino]-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015470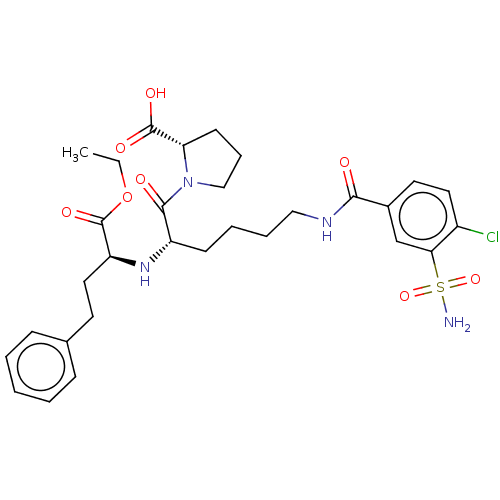 (1-[6-(4-Chloro-3-sulfamoyl-benzoylamino)-2-(1-etho...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021342 (3-(2-Mercapto-propionyl)-2,2,5,5-tetramethyl-thiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015481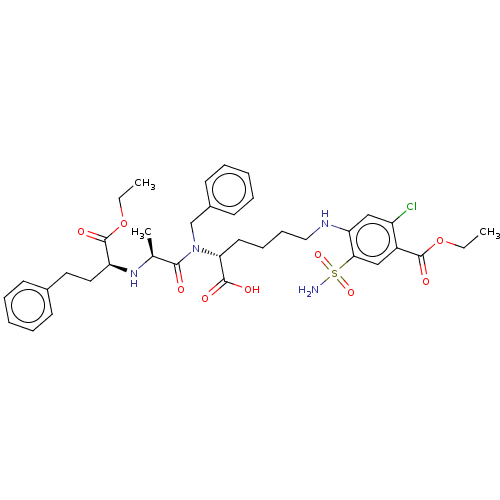 (4-(5-{Benzyl-[2-(1-ethoxycarbonyl-3-phenyl-propyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015468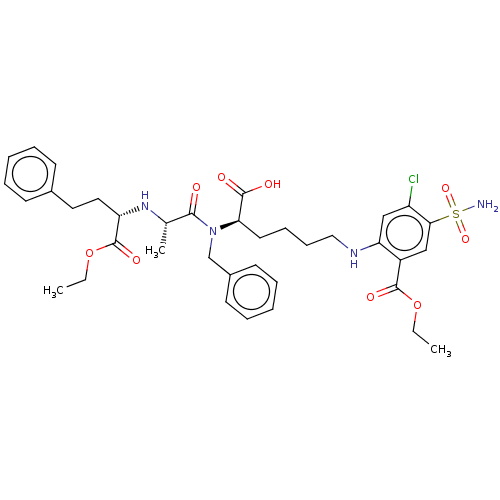 (2-(5-{Benzyl-[2-(1-ethoxycarbonyl-3-phenyl-propyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50421758 (CHEMBL2110310) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015480 (2-{1-[Carboxymethyl-(6-chloro-1,1-dioxo-7-sulfamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015475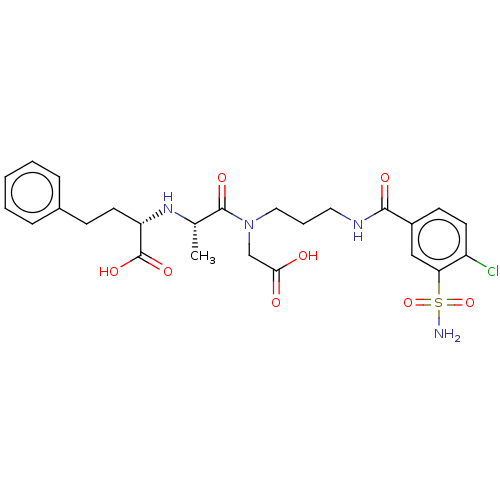 (2-(1-{Carboxymethyl-[3-(4-chloro-3-sulfamoyl-benzo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50368114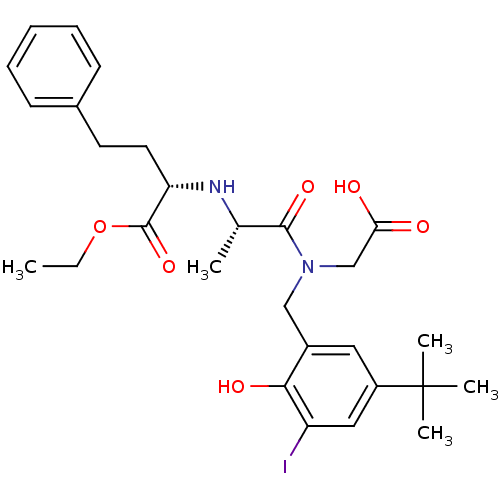 (CHEMBL1202591) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021341 (2-[(2-Acetylsulfanyl-propionyl)-cyclopentyl-amino]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50368116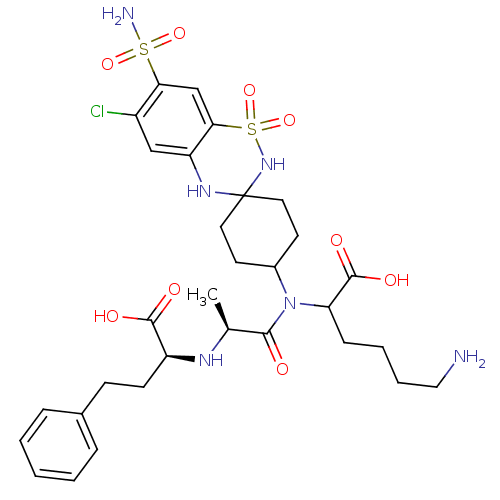 (CHEMBL1202158) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme(ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021337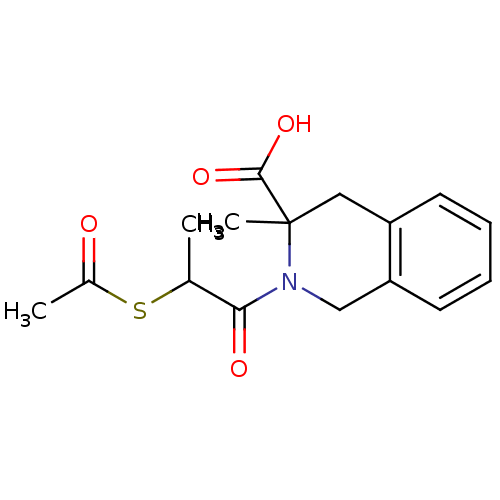 (2-(2-Acetylsulfanyl-propionyl)-3-methyl-1,2,3,4-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021335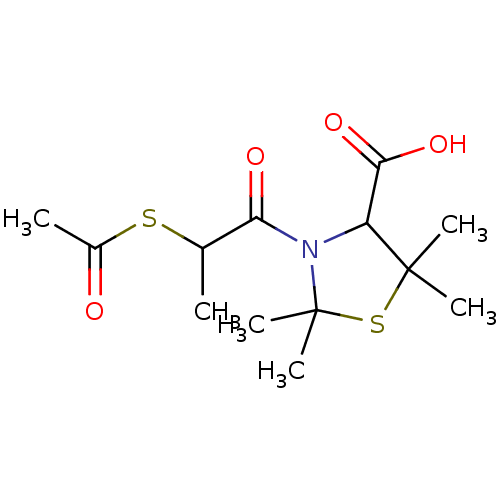 (3-(2-Acetylsulfanyl-propionyl)-2,2,5,5-tetramethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||