 Found 187 hits with Last Name = 'bass' and Initial = 'jy'
Found 187 hits with Last Name = 'bass' and Initial = 'jy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50336641

(6-[4-({[3-(2,6-Dichlorophenyl)-5-(1-methylethyl)-4...)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2nc(ccc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(40.05,8.98,;39.74,7.47,;38.28,6.98,;40.89,6.44,;42.4,6.77,;43.17,5.44,;42.15,4.29,;40.74,4.91,;39.41,4.14,;38.07,4.9,;36.74,4.13,;36.75,2.58,;35.41,1.81,;34.08,2.58,;34.08,4.12,;35.41,4.89,;32.74,1.81,;32.75,.27,;31.42,-.5,;30.08,.27,;28.75,-.5,;27.42,.27,;27.41,1.81,;28.75,2.58,;30.08,1.81,;31.41,2.58,;26.09,-.51,;24.75,.26,;26.09,-2.05,;42.47,2.79,;43.93,2.32,;45.07,3.35,;44.26,.82,;43.11,-.22,;41.64,.25,;41.32,1.76,;39.86,2.23,)| Show InChI InChI=1S/C29H22Cl2N2O4/c1-16(2)28-21(27(33-37-28)26-22(30)4-3-5-23(26)31)15-36-20-10-6-17(7-11-20)18-8-12-24-19(14-18)9-13-25(32-24)29(34)35/h3-14,16H,15H2,1-2H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome |
Bioorg Med Chem Lett 21: 1206-13 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.089
BindingDB Entry DOI: 10.7270/Q27D2VDD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM22164
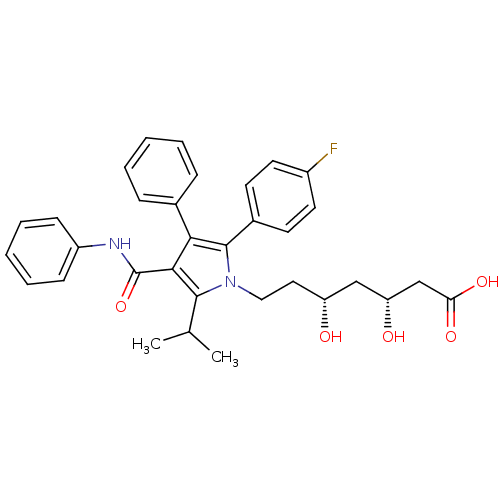
((3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylca...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H](O)C[C@@H](O)CC(O)=O)-c1ccccc1 |r| Show InChI InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 21: 1206-13 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.089
BindingDB Entry DOI: 10.7270/Q27D2VDD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336640

((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...)Show SMILES COC(=O)C1C(C(C(=O)OC)=C(C)N=C1C)c1ccccc1[N+]([O-])=O |c:13,t:10| Show InChI InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,13,15H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 21: 1206-13 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.089
BindingDB Entry DOI: 10.7270/Q27D2VDD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50336641

(6-[4-({[3-(2,6-Dichlorophenyl)-5-(1-methylethyl)-4...)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2nc(ccc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(40.05,8.98,;39.74,7.47,;38.28,6.98,;40.89,6.44,;42.4,6.77,;43.17,5.44,;42.15,4.29,;40.74,4.91,;39.41,4.14,;38.07,4.9,;36.74,4.13,;36.75,2.58,;35.41,1.81,;34.08,2.58,;34.08,4.12,;35.41,4.89,;32.74,1.81,;32.75,.27,;31.42,-.5,;30.08,.27,;28.75,-.5,;27.42,.27,;27.41,1.81,;28.75,2.58,;30.08,1.81,;31.41,2.58,;26.09,-.51,;24.75,.26,;26.09,-2.05,;42.47,2.79,;43.93,2.32,;45.07,3.35,;44.26,.82,;43.11,-.22,;41.64,.25,;41.32,1.76,;39.86,2.23,)| Show InChI InChI=1S/C29H22Cl2N2O4/c1-16(2)28-21(27(33-37-28)26-22(30)4-3-5-23(26)31)15-36-20-10-6-17(7-11-20)18-8-12-24-19(14-18)9-13-25(32-24)29(34)35/h3-14,16H,15H2,1-2H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsome |
Bioorg Med Chem Lett 21: 1206-13 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.089
BindingDB Entry DOI: 10.7270/Q27D2VDD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM21363
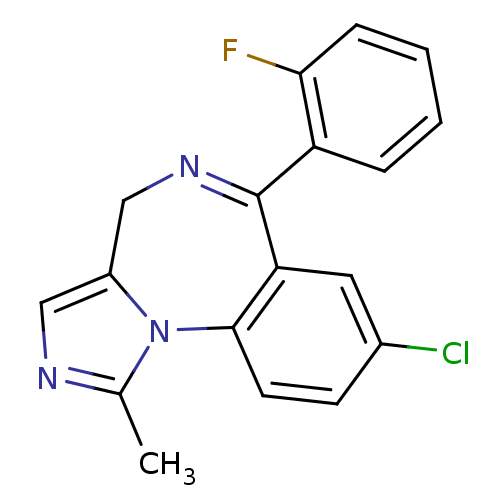
(12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,8-triaza...)Show SMILES Cc1ncc2CN=C(c3ccccc3F)c3cc(Cl)ccc3-n12 |t:6| Show InChI InChI=1S/C18H13ClFN3/c1-11-21-9-13-10-22-18(14-4-2-3-5-16(14)20)15-8-12(19)6-7-17(15)23(11)13/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 21: 1206-13 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.089
BindingDB Entry DOI: 10.7270/Q27D2VDD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50336641

(6-[4-({[3-(2,6-Dichlorophenyl)-5-(1-methylethyl)-4...)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2nc(ccc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(40.05,8.98,;39.74,7.47,;38.28,6.98,;40.89,6.44,;42.4,6.77,;43.17,5.44,;42.15,4.29,;40.74,4.91,;39.41,4.14,;38.07,4.9,;36.74,4.13,;36.75,2.58,;35.41,1.81,;34.08,2.58,;34.08,4.12,;35.41,4.89,;32.74,1.81,;32.75,.27,;31.42,-.5,;30.08,.27,;28.75,-.5,;27.42,.27,;27.41,1.81,;28.75,2.58,;30.08,1.81,;31.41,2.58,;26.09,-.51,;24.75,.26,;26.09,-2.05,;42.47,2.79,;43.93,2.32,;45.07,3.35,;44.26,.82,;43.11,-.22,;41.64,.25,;41.32,1.76,;39.86,2.23,)| Show InChI InChI=1S/C29H22Cl2N2O4/c1-16(2)28-21(27(33-37-28)26-22(30)4-3-5-23(26)31)15-36-20-10-6-17(7-11-20)18-8-12-24-19(14-18)9-13-25(32-24)29(34)35/h3-14,16H,15H2,1-2H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsome |
Bioorg Med Chem Lett 21: 1206-13 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.089
BindingDB Entry DOI: 10.7270/Q27D2VDD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336641

(6-[4-({[3-(2,6-Dichlorophenyl)-5-(1-methylethyl)-4...)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2nc(ccc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(40.05,8.98,;39.74,7.47,;38.28,6.98,;40.89,6.44,;42.4,6.77,;43.17,5.44,;42.15,4.29,;40.74,4.91,;39.41,4.14,;38.07,4.9,;36.74,4.13,;36.75,2.58,;35.41,1.81,;34.08,2.58,;34.08,4.12,;35.41,4.89,;32.74,1.81,;32.75,.27,;31.42,-.5,;30.08,.27,;28.75,-.5,;27.42,.27,;27.41,1.81,;28.75,2.58,;30.08,1.81,;31.41,2.58,;26.09,-.51,;24.75,.26,;26.09,-2.05,;42.47,2.79,;43.93,2.32,;45.07,3.35,;44.26,.82,;43.11,-.22,;41.64,.25,;41.32,1.76,;39.86,2.23,)| Show InChI InChI=1S/C29H22Cl2N2O4/c1-16(2)28-21(27(33-37-28)26-22(30)4-3-5-23(26)31)15-36-20-10-6-17(7-11-20)18-8-12-24-19(14-18)9-13-25(32-24)29(34)35/h3-14,16H,15H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsome |
Bioorg Med Chem Lett 21: 1206-13 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.089
BindingDB Entry DOI: 10.7270/Q27D2VDD |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 59 | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay measures ligand-mediated interaction of the SRC-1 peptide with the FXR ligand binding domain, using biotinylated FXR LBD coupled to allophy... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM30329

(Naphthoic acid-based analog, 1b)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2c(cccc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(-3.6,6.71,;-2.16,7.25,;-1.91,8.77,;-.97,6.27,;.52,6.66,;1.35,5.37,;.38,4.18,;-1.06,4.74,;-2.39,3.97,;-3.73,4.74,;-5.06,3.97,;-6.39,4.74,;-7.73,3.97,;-7.73,2.43,;-6.39,1.66,;-5.06,2.43,;-9.06,1.66,;-9.06,.12,;-10.39,-.65,;-11.73,.12,;-13.06,-.65,;-14.39,.12,;-14.39,1.66,;-13.06,2.43,;-11.73,1.66,;-10.39,2.43,;-13.06,-2.19,;-14.39,-2.96,;-11.73,-2.96,;.76,2.69,;-.28,1.55,;-1.78,1.89,;.18,.08,;1.68,-.26,;2.73,.88,;2.27,2.35,;3.31,3.48,)| Show InChI InChI=1S/C30H23Cl2NO4/c1-17(2)29-24(28(33-37-29)27-25(31)7-4-8-26(27)32)16-36-21-12-9-18(10-13-21)19-11-14-22-20(15-19)5-3-6-23(22)30(34)35/h3-15,17H,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 87 | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay measures ligand-mediated interaction of the SRC-1 peptide with the FXR ligand binding domain, using biotinylated FXR LBD coupled to allophy... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM30330
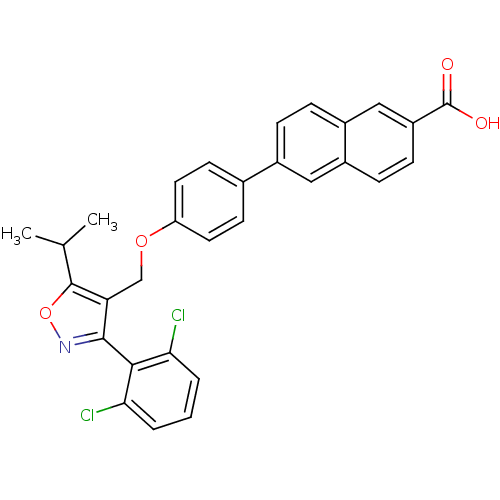
(Naphthoic acid-based analog, 1c)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2cc(ccc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(-3.6,6.71,;-2.16,7.25,;-1.91,8.77,;-.97,6.27,;.52,6.66,;1.35,5.37,;.38,4.18,;-1.06,4.74,;-2.39,3.97,;-3.73,4.74,;-5.06,3.97,;-6.39,4.74,;-7.73,3.97,;-7.73,2.43,;-6.39,1.66,;-5.06,2.43,;-9.06,1.66,;-9.06,.12,;-10.39,-.65,;-11.73,.12,;-13.06,-.65,;-14.39,.12,;-14.39,1.66,;-13.06,2.43,;-11.73,1.66,;-10.39,2.43,;-15.73,-.65,;-17.06,.12,;-15.73,-2.19,;.76,2.69,;-.28,1.55,;-1.78,1.89,;.18,.08,;1.68,-.26,;2.73,.88,;2.27,2.35,;3.31,3.48,)| Show InChI InChI=1S/C30H23Cl2NO4/c1-17(2)29-24(28(33-37-29)27-25(31)4-3-5-26(27)32)16-36-23-12-10-18(11-13-23)19-6-7-21-15-22(30(34)35)9-8-20(21)14-19/h3-15,17H,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay measures ligand-mediated interaction of the SRC-1 peptide with the FXR ligand binding domain, using biotinylated FXR LBD coupled to allophy... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM30331
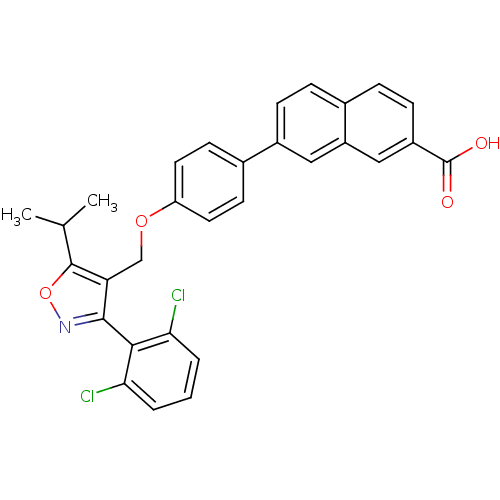
(Naphthoic acid-based analog, 1d)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2ccc(cc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(-3.6,6.71,;-2.16,7.25,;-1.91,8.77,;-.97,6.27,;.52,6.66,;1.35,5.37,;.38,4.18,;-1.06,4.74,;-2.39,3.97,;-3.73,4.74,;-5.06,3.97,;-6.39,4.74,;-7.73,3.97,;-7.73,2.43,;-6.39,1.66,;-5.06,2.43,;-9.06,1.66,;-9.06,.12,;-10.39,-.65,;-11.73,.12,;-13.06,-.65,;-14.39,.12,;-14.39,1.66,;-13.06,2.43,;-11.73,1.66,;-10.39,2.43,;-15.73,2.43,;-17.06,1.66,;-15.73,3.97,;.76,2.69,;-.28,1.55,;-1.78,1.89,;.18,.08,;1.68,-.26,;2.73,.88,;2.27,2.35,;3.31,3.48,)| Show InChI InChI=1S/C30H23Cl2NO4/c1-17(2)29-24(28(33-37-29)27-25(31)4-3-5-26(27)32)16-36-23-12-10-18(11-13-23)20-8-6-19-7-9-21(30(34)35)15-22(19)14-20/h3-15,17H,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 420 | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay measures ligand-mediated interaction of the SRC-1 peptide with the FXR ligand binding domain, using biotinylated FXR LBD coupled to allophy... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM30332
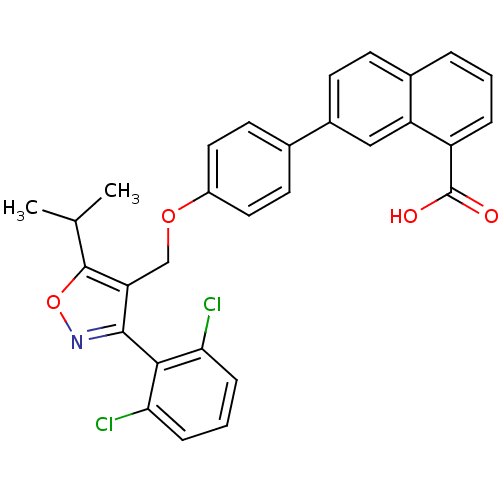
(Naphthoic acid-based analog, 1e)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2cccc(C(O)=O)c2c1)-c1c(Cl)cccc1Cl |(-3.6,6.71,;-2.16,7.25,;-1.91,8.77,;-.97,6.27,;.52,6.66,;1.35,5.37,;.38,4.18,;-1.06,4.74,;-2.39,3.97,;-3.73,4.74,;-5.06,3.97,;-6.39,4.74,;-7.73,3.97,;-7.73,2.43,;-6.39,1.66,;-5.06,2.43,;-9.06,1.66,;-9.06,.12,;-10.39,-.65,;-11.73,.12,;-13.06,-.65,;-14.39,.12,;-14.39,1.66,;-13.06,2.43,;-13.06,3.97,;-14.39,4.74,;-11.73,4.74,;-11.73,1.66,;-10.39,2.43,;.76,2.69,;-.28,1.55,;-1.78,1.89,;.18,.08,;1.68,-.26,;2.73,.88,;2.27,2.35,;3.31,3.48,)| Show InChI InChI=1S/C30H23Cl2NO4/c1-17(2)29-24(28(33-37-29)27-25(31)7-4-8-26(27)32)16-36-21-13-11-18(12-14-21)20-10-9-19-5-3-6-22(30(34)35)23(19)15-20/h3-15,17H,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay measures ligand-mediated interaction of the SRC-1 peptide with the FXR ligand binding domain, using biotinylated FXR LBD coupled to allophy... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The cell-based assay measures the ligand-mediated luminescense resulting from FXR-induced transcription of a luciferase reporter. FXR and the lucifer... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM30329

(Naphthoic acid-based analog, 1b)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2c(cccc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(-3.6,6.71,;-2.16,7.25,;-1.91,8.77,;-.97,6.27,;.52,6.66,;1.35,5.37,;.38,4.18,;-1.06,4.74,;-2.39,3.97,;-3.73,4.74,;-5.06,3.97,;-6.39,4.74,;-7.73,3.97,;-7.73,2.43,;-6.39,1.66,;-5.06,2.43,;-9.06,1.66,;-9.06,.12,;-10.39,-.65,;-11.73,.12,;-13.06,-.65,;-14.39,.12,;-14.39,1.66,;-13.06,2.43,;-11.73,1.66,;-10.39,2.43,;-13.06,-2.19,;-14.39,-2.96,;-11.73,-2.96,;.76,2.69,;-.28,1.55,;-1.78,1.89,;.18,.08,;1.68,-.26,;2.73,.88,;2.27,2.35,;3.31,3.48,)| Show InChI InChI=1S/C30H23Cl2NO4/c1-17(2)29-24(28(33-37-29)27-25(31)7-4-8-26(27)32)16-36-21-12-9-18(10-13-21)19-11-14-22-20(15-19)5-3-6-23(22)30(34)35/h3-15,17H,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The cell-based assay measures the ligand-mediated luminescense resulting from FXR-induced transcription of a luciferase reporter. FXR and the lucifer... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM30330

(Naphthoic acid-based analog, 1c)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2cc(ccc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(-3.6,6.71,;-2.16,7.25,;-1.91,8.77,;-.97,6.27,;.52,6.66,;1.35,5.37,;.38,4.18,;-1.06,4.74,;-2.39,3.97,;-3.73,4.74,;-5.06,3.97,;-6.39,4.74,;-7.73,3.97,;-7.73,2.43,;-6.39,1.66,;-5.06,2.43,;-9.06,1.66,;-9.06,.12,;-10.39,-.65,;-11.73,.12,;-13.06,-.65,;-14.39,.12,;-14.39,1.66,;-13.06,2.43,;-11.73,1.66,;-10.39,2.43,;-15.73,-.65,;-17.06,.12,;-15.73,-2.19,;.76,2.69,;-.28,1.55,;-1.78,1.89,;.18,.08,;1.68,-.26,;2.73,.88,;2.27,2.35,;3.31,3.48,)| Show InChI InChI=1S/C30H23Cl2NO4/c1-17(2)29-24(28(33-37-29)27-25(31)4-3-5-26(27)32)16-36-23-12-10-18(11-13-23)19-6-7-21-15-22(30(34)35)9-8-20(21)14-19/h3-15,17H,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The cell-based assay measures the ligand-mediated luminescense resulting from FXR-induced transcription of a luciferase reporter. FXR and the lucifer... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM30331

(Naphthoic acid-based analog, 1d)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2ccc(cc2c1)C(O)=O)-c1c(Cl)cccc1Cl |(-3.6,6.71,;-2.16,7.25,;-1.91,8.77,;-.97,6.27,;.52,6.66,;1.35,5.37,;.38,4.18,;-1.06,4.74,;-2.39,3.97,;-3.73,4.74,;-5.06,3.97,;-6.39,4.74,;-7.73,3.97,;-7.73,2.43,;-6.39,1.66,;-5.06,2.43,;-9.06,1.66,;-9.06,.12,;-10.39,-.65,;-11.73,.12,;-13.06,-.65,;-14.39,.12,;-14.39,1.66,;-13.06,2.43,;-11.73,1.66,;-10.39,2.43,;-15.73,2.43,;-17.06,1.66,;-15.73,3.97,;.76,2.69,;-.28,1.55,;-1.78,1.89,;.18,.08,;1.68,-.26,;2.73,.88,;2.27,2.35,;3.31,3.48,)| Show InChI InChI=1S/C30H23Cl2NO4/c1-17(2)29-24(28(33-37-29)27-25(31)4-3-5-26(27)32)16-36-23-12-10-18(11-13-23)20-8-6-19-7-9-21(30(34)35)15-22(19)14-20/h3-15,17H,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The cell-based assay measures the ligand-mediated luminescense resulting from FXR-induced transcription of a luciferase reporter. FXR and the lucifer... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM30332

(Naphthoic acid-based analog, 1e)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2cccc(C(O)=O)c2c1)-c1c(Cl)cccc1Cl |(-3.6,6.71,;-2.16,7.25,;-1.91,8.77,;-.97,6.27,;.52,6.66,;1.35,5.37,;.38,4.18,;-1.06,4.74,;-2.39,3.97,;-3.73,4.74,;-5.06,3.97,;-6.39,4.74,;-7.73,3.97,;-7.73,2.43,;-6.39,1.66,;-5.06,2.43,;-9.06,1.66,;-9.06,.12,;-10.39,-.65,;-11.73,.12,;-13.06,-.65,;-14.39,.12,;-14.39,1.66,;-13.06,2.43,;-13.06,3.97,;-14.39,4.74,;-11.73,4.74,;-11.73,1.66,;-10.39,2.43,;.76,2.69,;-.28,1.55,;-1.78,1.89,;.18,.08,;1.68,-.26,;2.73,.88,;2.27,2.35,;3.31,3.48,)| Show InChI InChI=1S/C30H23Cl2NO4/c1-17(2)29-24(28(33-37-29)27-25(31)7-4-8-26(27)32)16-36-21-13-11-18(12-14-21)20-10-9-19-5-3-6-22(30(34)35)23(19)15-20/h3-15,17H,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The cell-based assay measures the ligand-mediated luminescense resulting from FXR-induced transcription of a luciferase reporter. FXR and the lucifer... |
Bioorg Med Chem Lett 18: 4339-43 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.073
BindingDB Entry DOI: 10.7270/Q2KK994P |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258586
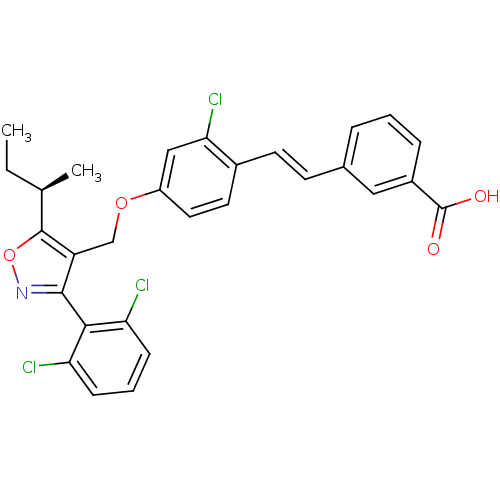
((R)-3-(4-((5-sec-butyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CC[C@@H](C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |r,wD:2.2,(33.96,-41.84,;32.5,-42.33,;32.19,-43.84,;30.73,-44.33,;33.35,-44.86,;34.85,-44.53,;35.63,-45.86,;34.6,-47.01,;33.2,-46.39,;31.87,-47.16,;30.54,-46.4,;29.21,-47.18,;27.86,-46.41,;26.54,-47.19,;26.55,-48.72,;25.22,-49.5,;23.88,-48.73,;22.55,-49.51,;22.55,-51.06,;21.22,-51.83,;19.88,-51.06,;19.89,-49.51,;21.21,-48.74,;18.55,-48.75,;18.55,-47.21,;17.21,-49.52,;27.88,-49.49,;27.88,-51.03,;29.21,-48.72,;34.93,-48.51,;33.78,-49.54,;32.32,-49.07,;34.1,-51.04,;35.57,-51.52,;36.72,-50.48,;36.39,-48.98,;37.53,-47.94,)| Show InChI InChI=1S/C29H24Cl3NO4/c1-3-17(2)28-22(27(33-37-28)26-23(30)8-5-9-24(26)31)16-36-21-13-12-19(25(32)15-21)11-10-18-6-4-7-20(14-18)29(34)35/h4-15,17H,3,16H2,1-2H3,(H,34,35)/b11-10+/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 105 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258558
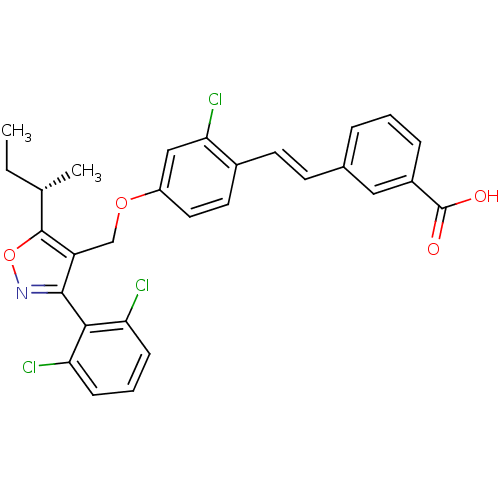
((S)-3-(4-((5-sec-butyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CC[C@H](C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |r,wU:2.2,(10.7,-43.27,;9.24,-43.76,;8.93,-45.27,;7.47,-45.76,;10.08,-46.29,;11.59,-45.96,;12.36,-47.29,;11.34,-48.44,;9.94,-47.82,;8.61,-48.59,;7.27,-47.83,;5.94,-48.61,;4.6,-47.84,;3.27,-48.62,;3.28,-50.15,;1.95,-50.93,;.62,-50.16,;-.71,-50.94,;-.71,-52.49,;-2.05,-53.26,;-3.38,-52.49,;-3.38,-50.94,;-2.05,-50.17,;-4.72,-50.18,;-4.72,-48.64,;-6.05,-50.95,;4.62,-50.92,;4.62,-52.46,;5.95,-50.15,;11.66,-49.94,;10.52,-50.97,;9.05,-50.5,;10.84,-52.48,;12.31,-52.95,;13.45,-51.91,;13.12,-50.41,;14.26,-49.37,)| Show InChI InChI=1S/C29H24Cl3NO4/c1-3-17(2)28-22(27(33-37-28)26-23(30)8-5-9-24(26)31)16-36-21-13-12-19(25(32)15-21)11-10-18-6-4-7-20(14-18)29(34)35/h4-15,17H,3,16H2,1-2H3,(H,34,35)/b11-10+/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258557
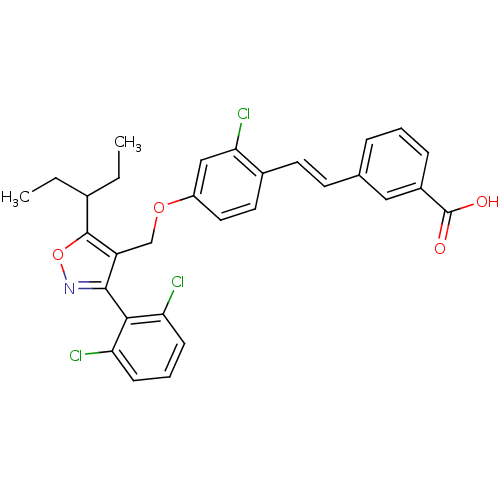
(3-(2-chloro-4-((3-(2,6-dichlorophenyl)-5-(pentan-3...)Show SMILES CCC(CC)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(32.14,-25.41,;33.29,-26.43,;32.99,-27.94,;31.53,-28.43,;30.37,-27.41,;34.14,-28.96,;35.64,-28.63,;36.42,-29.96,;35.4,-31.11,;34,-30.49,;32.67,-31.27,;31.33,-30.5,;30,-31.28,;28.65,-30.51,;27.33,-31.29,;27.34,-32.82,;26.01,-33.6,;24.67,-32.83,;23.34,-33.61,;23.35,-35.16,;22.01,-35.93,;20.68,-35.16,;20.68,-33.62,;22.01,-32.84,;19.34,-32.85,;19.34,-31.31,;18.01,-33.62,;28.67,-33.59,;28.68,-35.13,;30,-32.82,;35.72,-32.61,;34.57,-33.64,;33.11,-33.17,;34.9,-35.15,;36.36,-35.62,;37.51,-34.58,;37.18,-33.08,;38.32,-32.04,)| Show InChI InChI=1S/C30H26Cl3NO4/c1-3-19(4-2)29-23(28(34-38-29)27-24(31)9-6-10-25(27)32)17-37-22-14-13-20(26(33)16-22)12-11-18-7-5-8-21(15-18)30(35)36/h5-16,19H,3-4,17H2,1-2H3,(H,35,36)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258556

(3-(2-chloro-4-((5-cyclopentyl-3-(2,6-dichloropheny...)Show SMILES OC(=O)c1cccc(\C=C\c2ccc(OCc3c(noc3C3CCCC3)-c3c(Cl)cccc3Cl)cc2Cl)c1 |(-6.07,-30.87,;-6.07,-32.41,;-7.4,-33.18,;-4.73,-33.18,;-4.73,-34.72,;-3.4,-35.5,;-2.06,-34.72,;-2.07,-33.17,;-.74,-32.4,;.6,-33.16,;1.93,-32.39,;1.92,-30.86,;3.24,-30.08,;4.59,-30.84,;5.92,-30.07,;7.26,-30.83,;8.59,-30.05,;9.99,-30.68,;11.01,-29.53,;10.23,-28.2,;8.73,-28.53,;7.57,-27.5,;7.72,-25.97,;6.31,-25.36,;5.29,-26.51,;6.07,-27.84,;10.31,-32.17,;9.16,-33.21,;7.7,-32.73,;9.49,-34.71,;10.95,-35.18,;12.1,-34.14,;11.77,-32.64,;12.91,-31.61,;4.59,-32.39,;3.26,-33.16,;3.27,-34.7,;-3.4,-32.41,)| Show InChI InChI=1S/C30H24Cl3NO4/c31-24-9-4-10-25(32)27(24)28-23(29(38-34-28)20-6-1-2-7-20)17-37-22-14-13-19(26(33)16-22)12-11-18-5-3-8-21(15-18)30(35)36/h3-5,8-16,20H,1-2,6-7,17H2,(H,35,36)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258513
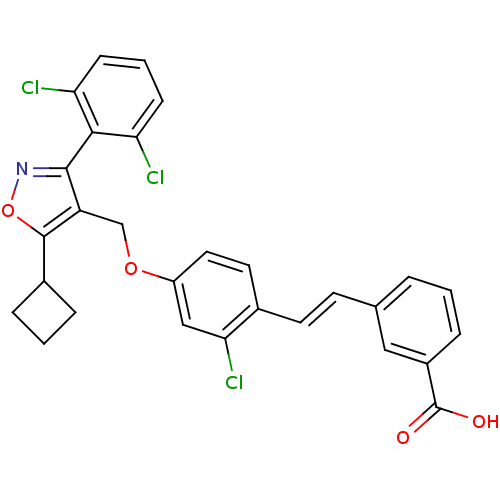
(3-(2-chloro-4-((5-cyclobutyl-3-(2,6-dichlorophenyl...)Show SMILES OC(=O)c1cccc(\C=C\c2ccc(OCc3c(noc3C3CCC3)-c3c(Cl)cccc3Cl)cc2Cl)c1 |(21.49,-15.58,;21.49,-17.12,;20.16,-17.89,;22.83,-17.89,;22.83,-19.43,;24.17,-20.2,;25.5,-19.43,;25.5,-17.88,;26.83,-17.11,;28.16,-17.87,;29.5,-17.1,;29.48,-15.57,;30.81,-14.79,;32.15,-15.55,;33.48,-14.78,;34.82,-15.54,;36.15,-14.76,;37.55,-15.39,;38.57,-14.24,;37.8,-12.91,;36.29,-13.23,;35.14,-12.22,;35.05,-10.68,;33.51,-10.77,;33.6,-12.31,;37.87,-16.88,;36.73,-17.92,;35.26,-17.44,;37.05,-19.42,;38.52,-19.89,;39.66,-18.85,;39.33,-17.35,;40.47,-16.31,;32.16,-17.09,;30.83,-17.87,;30.83,-19.41,;24.16,-17.12,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-8-3-9-24(31)26(23)27-22(28(37-33-27)19-5-2-6-19)16-36-21-13-12-18(25(32)15-21)11-10-17-4-1-7-20(14-17)29(34)35/h1,3-4,7-15,19H,2,5-6,16H2,(H,34,35)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258512
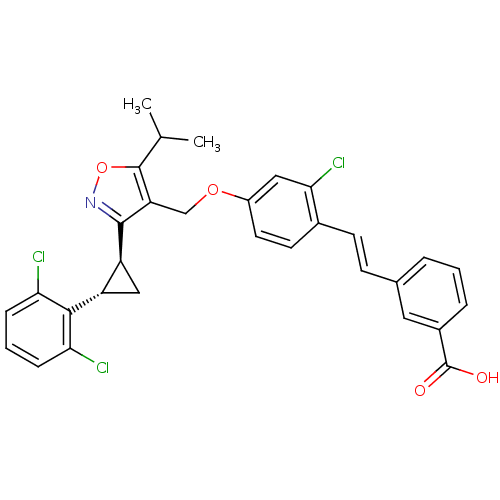
(3-(2-chloro-4-((3-((trans)-2-(2,6-dichlorophenyl)c...)Show SMILES CC(C)c1onc([C@H]2C[C@@H]2c2c(Cl)cccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 |r| Show InChI InChI=1S/C31H26Cl3NO4/c1-17(2)30-24(29(35-39-30)23-15-22(23)28-25(32)7-4-8-26(28)33)16-38-21-12-11-19(27(34)14-21)10-9-18-5-3-6-20(13-18)31(36)37/h3-14,17,22-23H,15-16H2,1-2H3,(H,36,37)/b10-9+/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258511
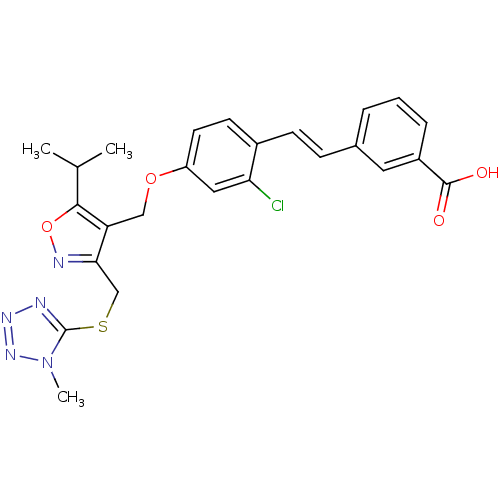
(3-(2-chloro-4-((3-(2,6-dichlorophenyl)-5-(1-methyl...)Show SMILES CC(C)c1onc(CSc2nnnn2C)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C25H24ClN5O4S/c1-15(2)23-20(22(28-35-23)14-36-25-27-29-30-31(25)3)13-34-19-10-9-17(21(26)12-19)8-7-16-5-4-6-18(11-16)24(32)33/h4-12,15H,13-14H2,1-3H3,(H,32,33)/b8-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 930 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258486
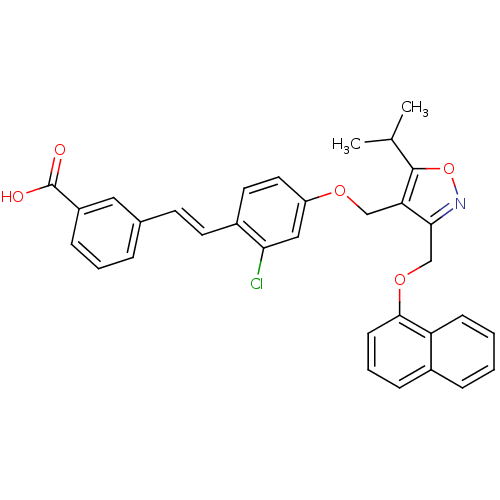
(3-(2-chloro-4-((5-isopropyl-3-((naphthalen-1-yloxy...)Show SMILES CC(C)c1onc(COc2cccc3ccccc23)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C33H28ClNO5/c1-21(2)32-28(30(35-40-32)20-39-31-12-6-9-23-8-3-4-11-27(23)31)19-38-26-16-15-24(29(34)18-26)14-13-22-7-5-10-25(17-22)33(36)37/h3-18,21H,19-20H2,1-2H3,(H,36,37)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 960 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258485
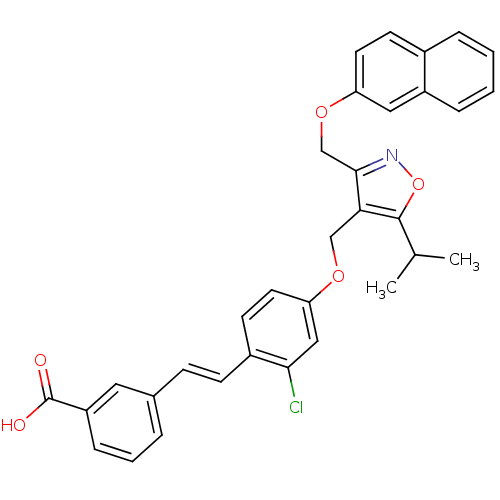
(3-(2-chloro-4-((5-isopropyl-3-((naphthalen-2-yloxy...)Show SMILES CC(C)c1onc(COc2ccc3ccccc3c2)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C33H28ClNO5/c1-21(2)32-29(31(35-40-32)20-39-27-14-12-23-7-3-4-8-25(23)17-27)19-38-28-15-13-24(30(34)18-28)11-10-22-6-5-9-26(16-22)33(36)37/h3-18,21H,19-20H2,1-2H3,(H,36,37)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 930 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258484
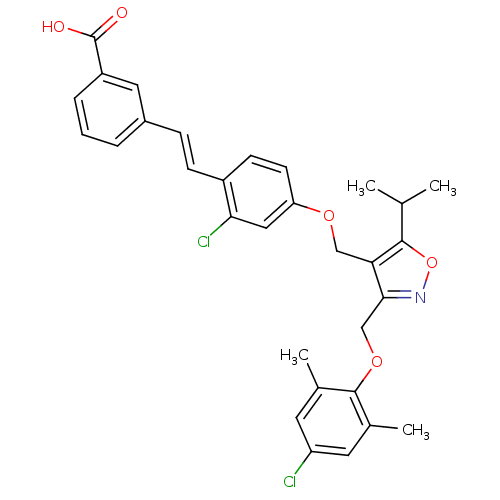
(3-(2-chloro-4-((3-((4-chloro-2,6-dimethylphenoxy)m...)Show SMILES CC(C)c1onc(COc2c(C)cc(Cl)cc2C)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C31H29Cl2NO5/c1-18(2)29-26(28(34-39-29)17-38-30-19(3)12-24(32)13-20(30)4)16-37-25-11-10-22(27(33)15-25)9-8-21-6-5-7-23(14-21)31(35)36/h5-15,18H,16-17H2,1-4H3,(H,35,36)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258453
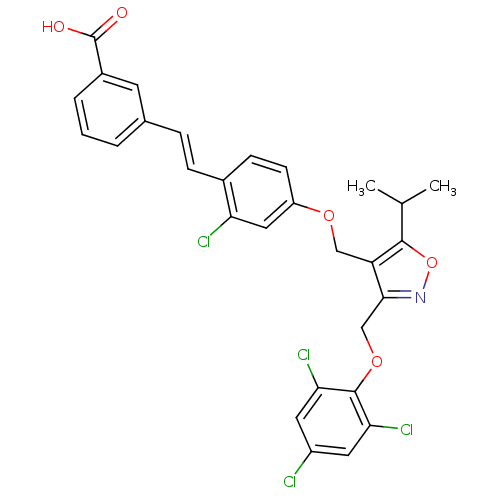
(3-(2-chloro-4-((5-isopropyl-3-((2,4,6-trichlorophe...)Show SMILES CC(C)c1onc(COc2c(Cl)cc(Cl)cc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H23Cl4NO5/c1-16(2)27-22(26(34-39-27)15-38-28-24(32)11-20(30)12-25(28)33)14-37-21-9-8-18(23(31)13-21)7-6-17-4-3-5-19(10-17)29(35)36/h3-13,16H,14-15H2,1-2H3,(H,35,36)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258452
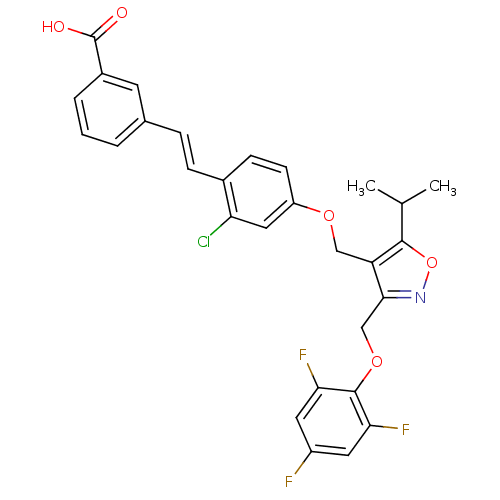
(3-(2-chloro-4-((5-isopropyl-3-((2,4,6-trifluorophe...)Show SMILES CC(C)c1onc(COc2c(F)cc(F)cc2F)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H23ClF3NO5/c1-16(2)27-22(26(34-39-27)15-38-28-24(32)11-20(31)12-25(28)33)14-37-21-9-8-18(23(30)13-21)7-6-17-4-3-5-19(10-17)29(35)36/h3-13,16H,14-15H2,1-2H3,(H,35,36)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258451
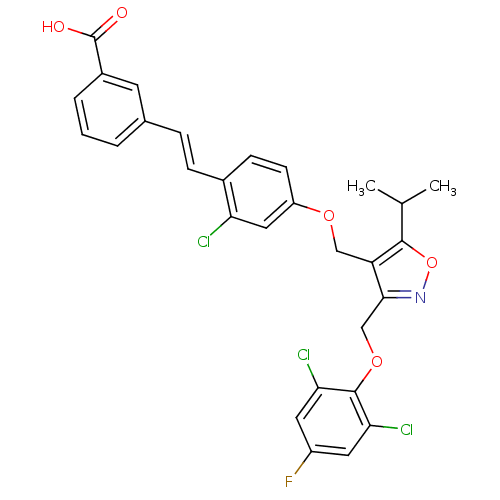
(3-(2-chloro-4-((3-((2,6-dichloro-4-fluorophenoxy)m...)Show SMILES CC(C)c1onc(COc2c(Cl)cc(F)cc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H23Cl3FNO5/c1-16(2)27-22(26(34-39-27)15-38-28-24(31)11-20(33)12-25(28)32)14-37-21-9-8-18(23(30)13-21)7-6-17-4-3-5-19(10-17)29(35)36/h3-13,16H,14-15H2,1-2H3,(H,35,36)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258423
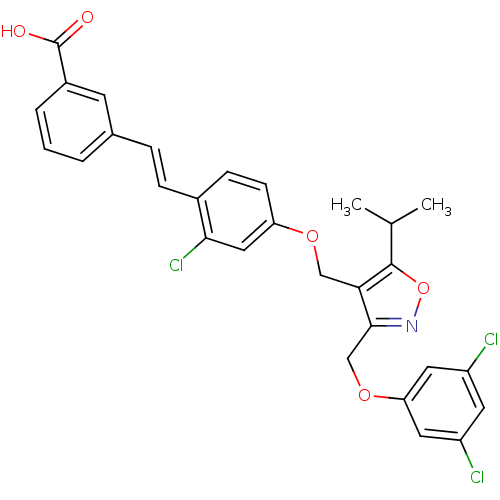
(3-(2-chloro-4-((3-((3,5-dichlorophenoxy)methyl)-5-...)Show SMILES CC(C)c1onc(COc2cc(Cl)cc(Cl)c2)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO5/c1-17(2)28-25(27(33-38-28)16-37-24-12-21(30)11-22(31)13-24)15-36-23-9-8-19(26(32)14-23)7-6-18-4-3-5-20(10-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258422
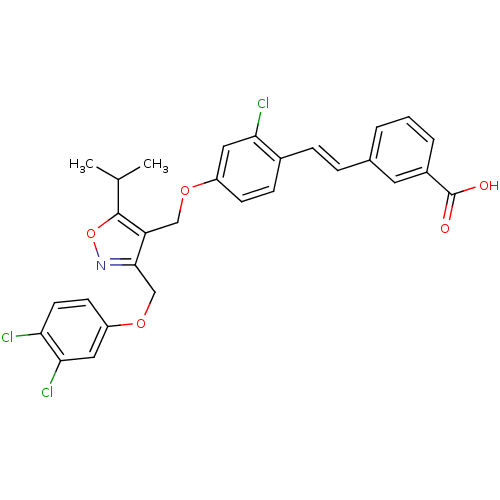
(3-(2-chloro-4-((3-((3,4-dichlorophenoxy)methyl)-5-...)Show SMILES CC(C)c1onc(COc2ccc(Cl)c(Cl)c2)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO5/c1-17(2)28-23(27(33-38-28)16-37-22-10-11-24(30)26(32)14-22)15-36-21-9-8-19(25(31)13-21)7-6-18-4-3-5-20(12-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258421
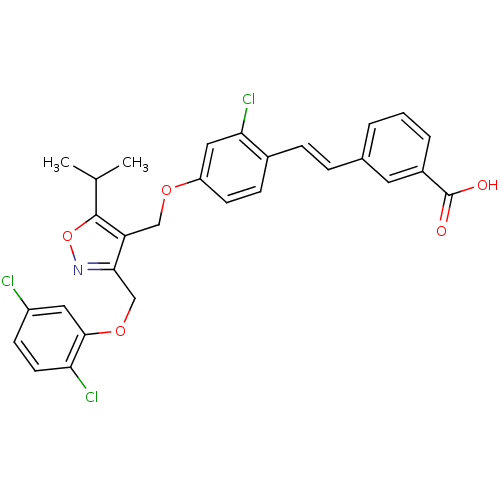
(3-(2-chloro-4-((3-((2,5-dichlorophenoxy)methyl)-5-...)Show SMILES CC(C)c1onc(COc2cc(Cl)ccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO5/c1-17(2)28-23(26(33-38-28)16-37-27-13-21(30)9-11-24(27)31)15-36-22-10-8-19(25(32)14-22)7-6-18-4-3-5-20(12-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258323
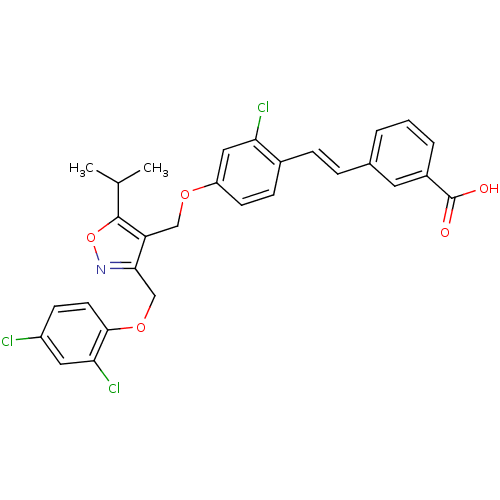
(3-(2-chloro-4-((3-((2,4-dichlorophenoxy)methyl)-5-...)Show SMILES CC(C)c1onc(COc2ccc(Cl)cc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO5/c1-17(2)28-23(26(33-38-28)16-37-27-11-9-21(30)13-25(27)32)15-36-22-10-8-19(24(31)14-22)7-6-18-4-3-5-20(12-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258283
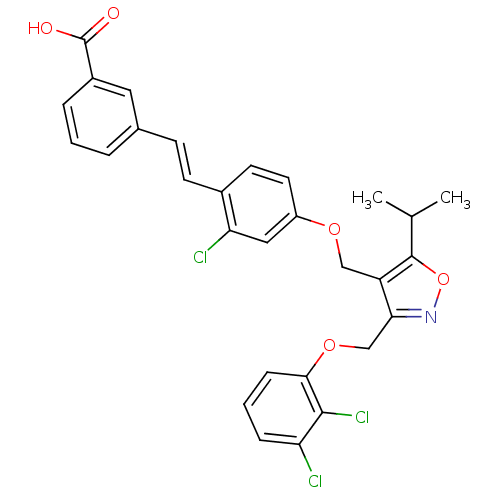
(3-(2-chloro-4-((3-((2,3-dichlorophenoxy)methyl)-5-...)Show SMILES CC(C)c1onc(COc2cccc(Cl)c2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO5/c1-17(2)28-22(25(33-38-28)16-37-26-8-4-7-23(30)27(26)32)15-36-21-12-11-19(24(31)14-21)10-9-18-5-3-6-20(13-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258282
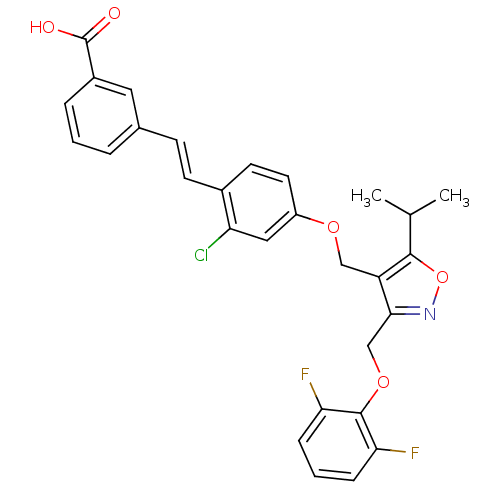
(3-(2-chloro-4-((3-((2,6-difluorophenoxy)methyl)-5-...)Show SMILES CC(C)c1onc(COc2c(F)cccc2F)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24ClF2NO5/c1-17(2)27-22(26(33-38-27)16-37-28-24(31)7-4-8-25(28)32)15-36-21-12-11-19(23(30)14-21)10-9-18-5-3-6-20(13-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258281
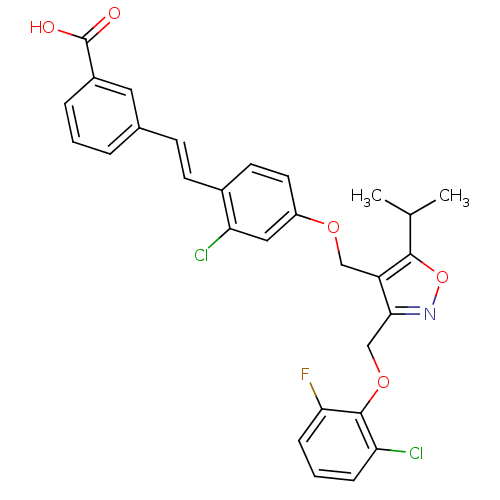
(3-(2-chloro-4-((3-((2-chloro-6-fluorophenoxy)methy...)Show SMILES CC(C)c1onc(COc2c(F)cccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl2FNO5/c1-17(2)27-22(26(33-38-27)16-37-28-23(30)7-4-8-25(28)32)15-36-21-12-11-19(24(31)14-21)10-9-18-5-3-6-20(13-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258852
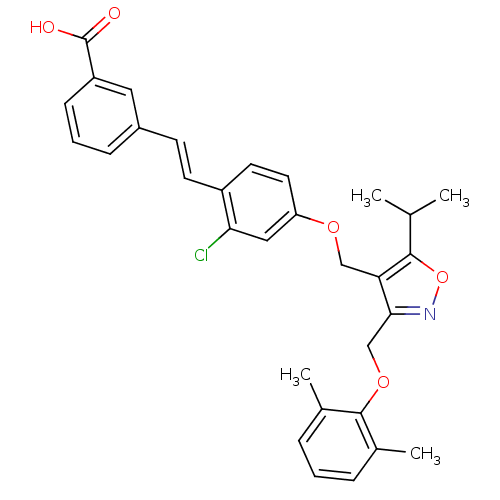
(3-(2-chloro-4-((3-((2,6-dimethylphenoxy)methyl)-5-...)Show SMILES CC(C)c1onc(COc2c(C)cccc2C)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C31H30ClNO5/c1-19(2)29-26(28(33-38-29)18-37-30-20(3)7-5-8-21(30)4)17-36-25-14-13-23(27(32)16-25)12-11-22-9-6-10-24(15-22)31(34)35/h5-16,19H,17-18H2,1-4H3,(H,34,35)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258851
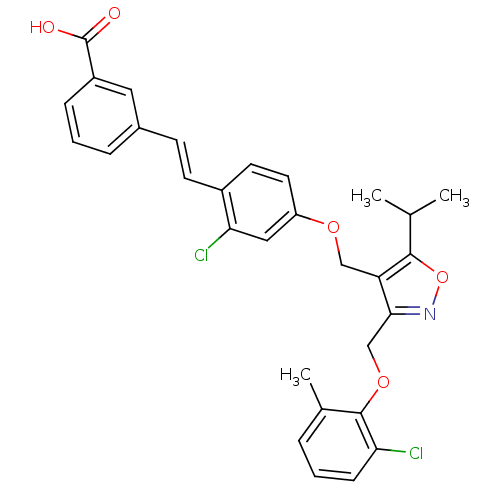
(3-(2-chloro-4-((3-((2-chloro-6-methylphenoxy)methy...)Show SMILES CC(C)c1onc(COc2c(C)cccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C30H27Cl2NO5/c1-18(2)28-24(27(33-38-28)17-37-29-19(3)6-4-9-25(29)31)16-36-23-13-12-21(26(32)15-23)11-10-20-7-5-8-22(14-20)30(34)35/h4-15,18H,16-17H2,1-3H3,(H,34,35)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258850
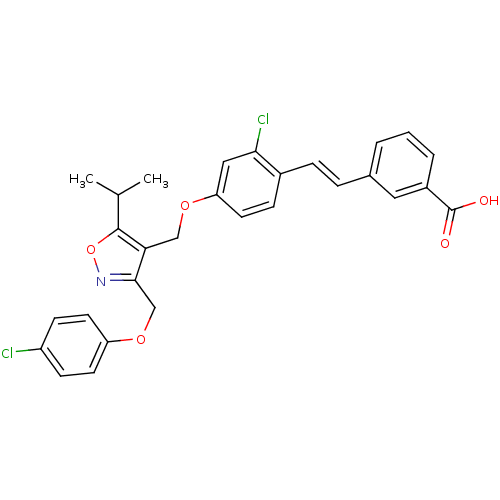
(3-(2-chloro-4-((3-((4-chlorophenoxy)methyl)-5-isop...)Show SMILES CC(C)c1onc(COc2ccc(Cl)cc2)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H25Cl2NO5/c1-18(2)28-25(27(32-37-28)17-36-23-12-9-22(30)10-13-23)16-35-24-11-8-20(26(31)15-24)7-6-19-4-3-5-21(14-19)29(33)34/h3-15,18H,16-17H2,1-2H3,(H,33,34)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258810
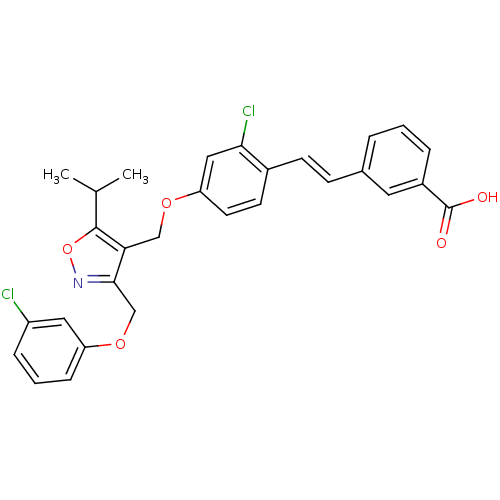
(3-(2-chloro-4-((3-((3-chlorophenoxy)methyl)-5-isop...)Show SMILES CC(C)c1onc(COc2cccc(Cl)c2)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H25Cl2NO5/c1-18(2)28-25(27(32-37-28)17-36-23-8-4-7-22(30)14-23)16-35-24-12-11-20(26(31)15-24)10-9-19-5-3-6-21(13-19)29(33)34/h3-15,18H,16-17H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258809
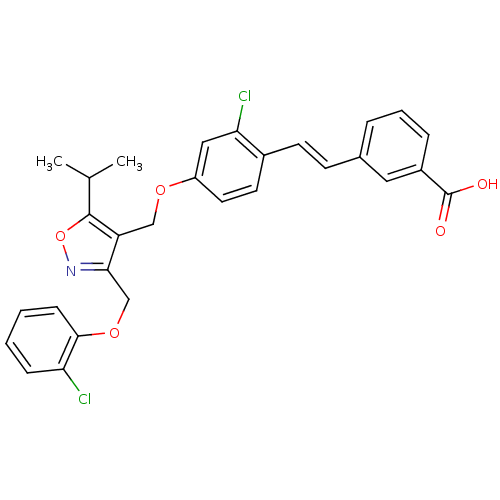
(3-(2-chloro-4-((3-((2-chlorophenoxy)methyl)-5-isop...)Show SMILES CC(C)c1onc(COc2ccccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H25Cl2NO5/c1-18(2)28-23(26(32-37-28)17-36-27-9-4-3-8-24(27)30)16-35-22-13-12-20(25(31)15-22)11-10-19-6-5-7-21(14-19)29(33)34/h3-15,18H,16-17H2,1-2H3,(H,33,34)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258808
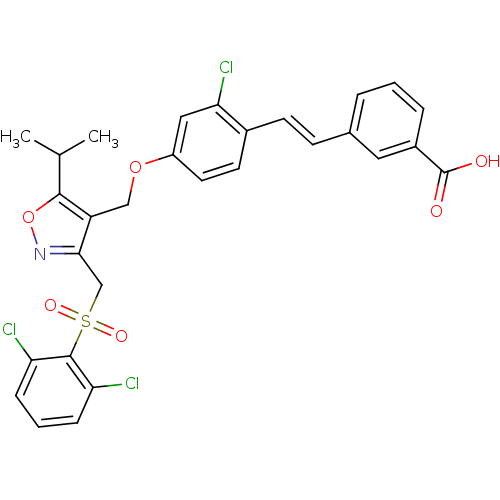
(3-(2-chloro-4-((3-((2,6-dichlorophenylsulfonyl)met...)Show SMILES CC(C)c1onc(CS(=O)(=O)c2c(Cl)cccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO6S/c1-17(2)27-22(26(33-39-27)16-40(36,37)28-23(30)7-4-8-24(28)31)15-38-21-12-11-19(25(32)14-21)10-9-18-5-3-6-20(13-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258778
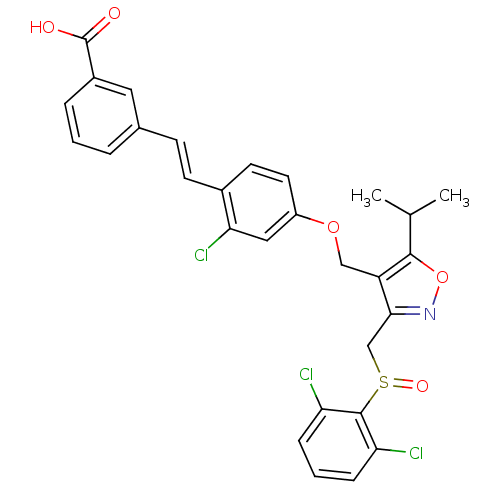
(3-(2-chloro-4-((3-((2,6-dichlorophenylsulfinyl)met...)Show SMILES CC(C)c1onc(CS(=O)c2c(Cl)cccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO5S/c1-17(2)27-22(26(33-38-27)16-39(36)28-23(30)7-4-8-24(28)31)15-37-21-12-11-19(25(32)14-21)10-9-18-5-3-6-20(13-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258777
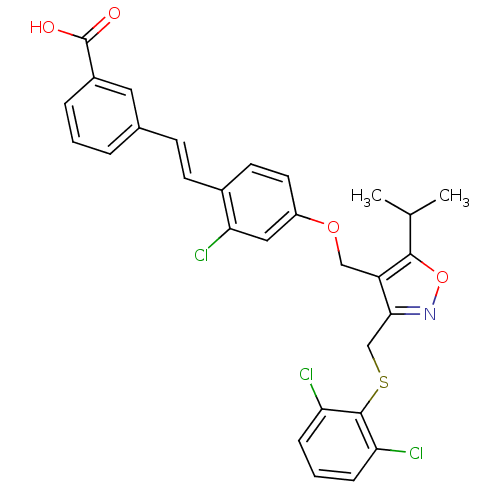
(3-(2-chloro-4-((3-((2,6-dichlorophenylthio)methyl)...)Show SMILES CC(C)c1onc(CSc2c(Cl)cccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO4S/c1-17(2)27-22(26(33-37-27)16-38-28-23(30)7-4-8-24(28)31)15-36-21-12-11-19(25(32)14-21)10-9-18-5-3-6-20(13-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258776
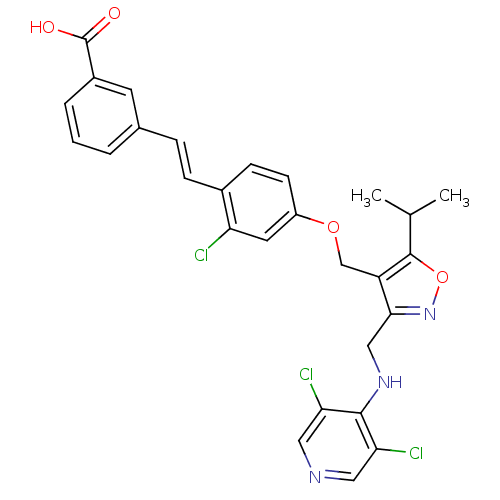
(3-(2-chloro-4-((3-((3,5-dichloropyridin-4-ylamino)...)Show SMILES CC(C)c1onc(CNc2c(Cl)cncc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C28H24Cl3N3O4/c1-16(2)27-21(25(34-38-27)14-33-26-23(30)12-32-13-24(26)31)15-37-20-9-8-18(22(29)11-20)7-6-17-4-3-5-19(10-17)28(35)36/h3-13,16H,14-15H2,1-2H3,(H,32,33)(H,35,36)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258746
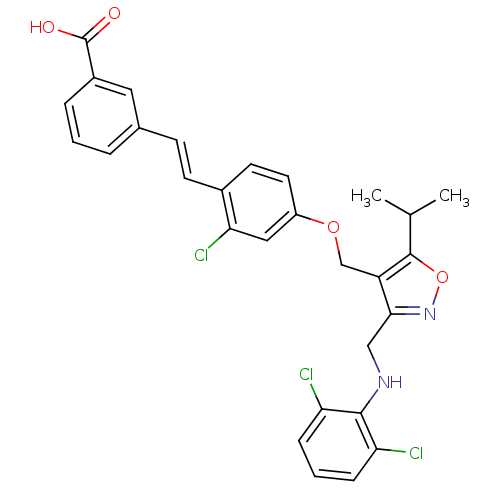
(3-(2-chloro-4-((3-((2,6-dichlorophenylamino)methyl...)Show SMILES CC(C)c1onc(CNc2c(Cl)cccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H25Cl3N2O4/c1-17(2)28-22(26(34-38-28)15-33-27-23(30)7-4-8-24(27)31)16-37-21-12-11-19(25(32)14-21)10-9-18-5-3-6-20(13-18)29(35)36/h3-14,17,33H,15-16H2,1-2H3,(H,35,36)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258745
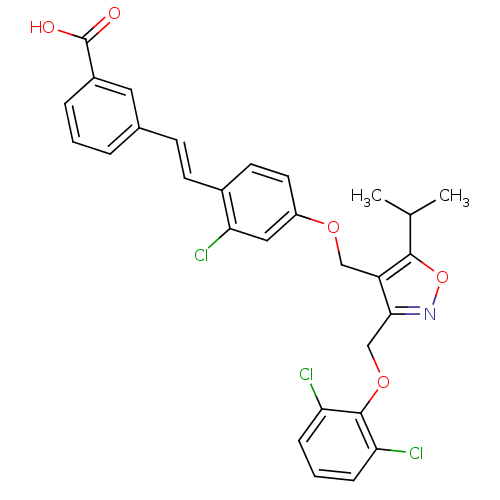
(3-(2-chloro-4-((3-((2,6-dichlorophenoxy)methyl)-5-...)Show SMILES CC(C)c1onc(COc2c(Cl)cccc2Cl)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H24Cl3NO5/c1-17(2)27-22(26(33-38-27)16-37-28-23(30)7-4-8-24(28)31)15-36-21-12-11-19(25(32)14-21)10-9-18-5-3-6-20(13-18)29(34)35/h3-14,17H,15-16H2,1-2H3,(H,34,35)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258744
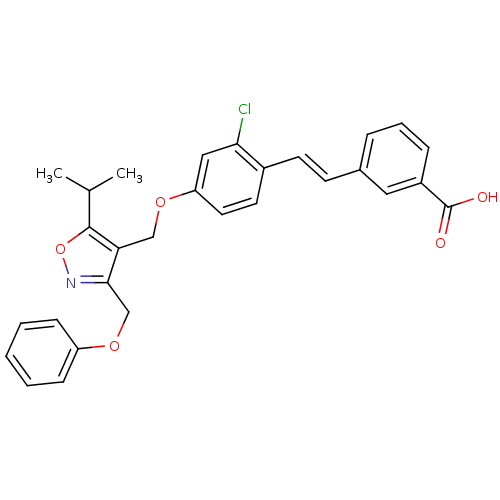
(3-(2-chloro-4-((5-isopropyl-3-(phenoxymethyl)isoxa...)Show SMILES CC(C)c1onc(COc2ccccc2)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C29H26ClNO5/c1-19(2)28-25(27(31-36-28)18-35-23-9-4-3-5-10-23)17-34-24-14-13-21(26(30)16-24)12-11-20-7-6-8-22(15-20)29(32)33/h3-16,19H,17-18H2,1-2H3,(H,32,33)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258713
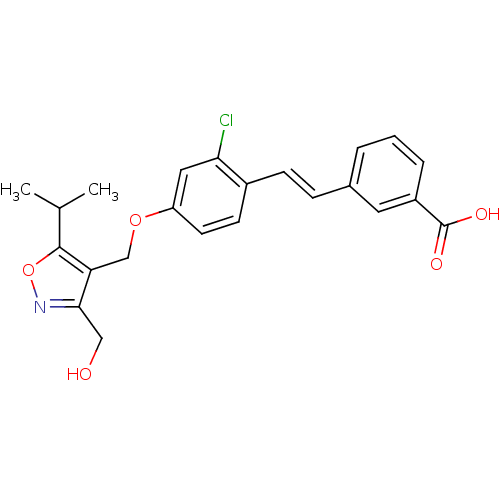
(3-(2-chloro-4-((3-(hydroxymethyl)-5-isopropylisoxa...)Show SMILES CC(C)c1onc(CO)c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H22ClNO5/c1-14(2)22-19(21(12-26)25-30-22)13-29-18-9-8-16(20(24)11-18)7-6-15-4-3-5-17(10-15)23(27)28/h3-11,14,26H,12-13H2,1-2H3,(H,27,28)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as SRC1 peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 19: 2969-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.047
BindingDB Entry DOI: 10.7270/Q2JH3M27 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data