

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2510 ((+/-)-3-[ 1-(Cyclopentylthio)-3-methylbutyl]-4-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 33 | -44.4 | 58 | n/a | n/a | n/a | n/a | 4.7 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | J Med Chem 38: 898-905 (1995) Article DOI: 10.1021/jm00006a007 BindingDB Entry DOI: 10.7270/Q2348HJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM3443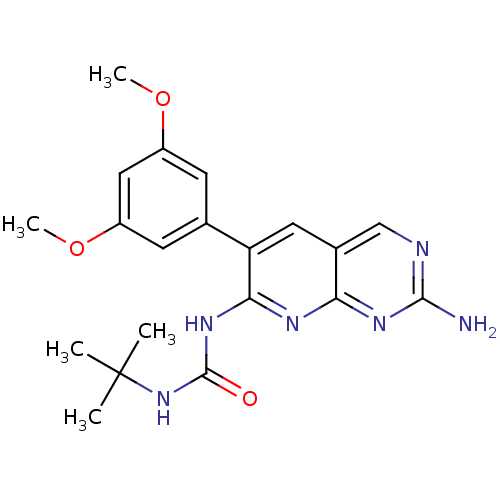 (1-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 45.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 286: 569-77 (1998) BindingDB Entry DOI: 10.7270/Q2C24TZQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008145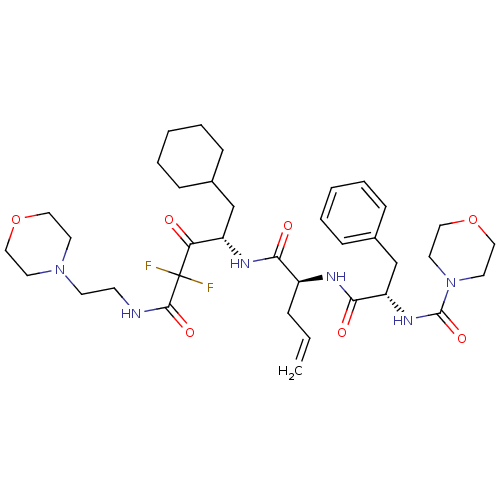 (CHEMBL286147 | Morpholine-4-carboxylic acid (1-{1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045292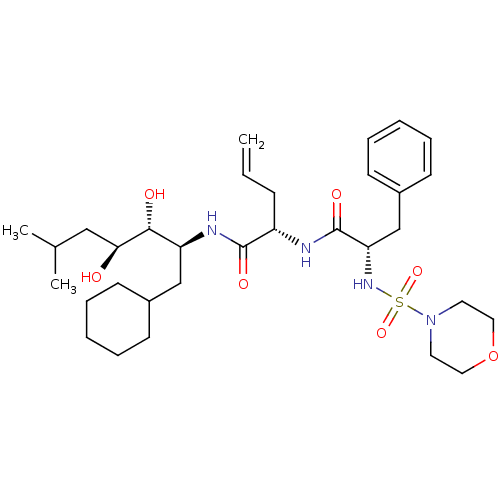 ((S)-2-[(S)-2-(Morpholine-4-sulfonylamino)-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045292 ((S)-2-[(S)-2-(Morpholine-4-sulfonylamino)-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for inhibitory activity against monkey plasma renin. | Bioorg Med Chem Lett 3: 2119-2124 (1993) Article DOI: 10.1016/S0960-894X(01)81029-5 BindingDB Entry DOI: 10.7270/Q25B02DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008141 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008130 (2-[3-Phenyl-2-(3-pyridin-3-yl-propionylamino)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008129 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008151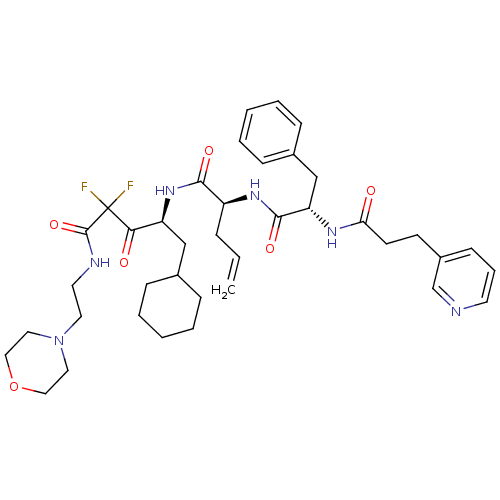 (2-[3-Phenyl-2-(3-pyridin-3-yl-propionylamino)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008138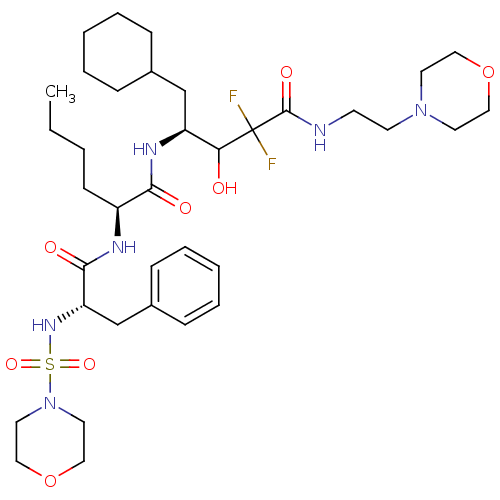 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008132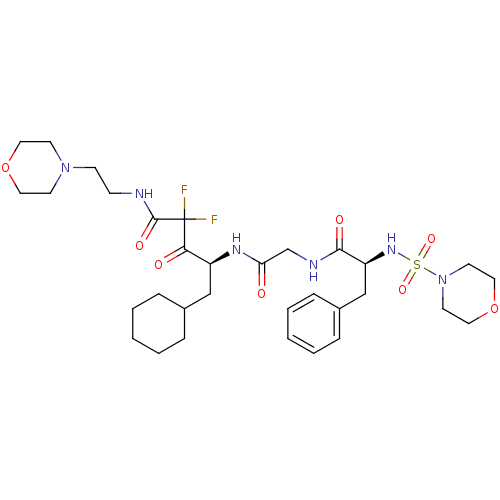 (5-Cyclohexyl-2,2-difluoro-4-{2-[2-(morpholine-4-su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008153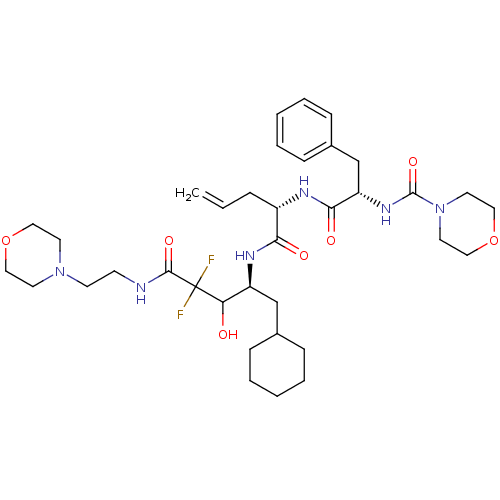 (CHEMBL32575 | Morpholine-4-carboxylic acid (1-{1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008147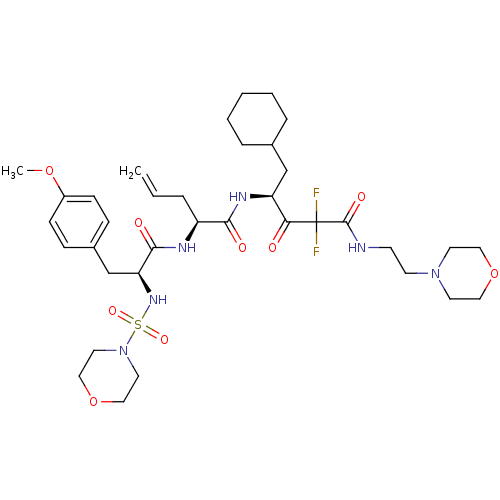 (2-[3-(4-Methoxy-phenyl)-2-(morpholine-4-sulfonylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008121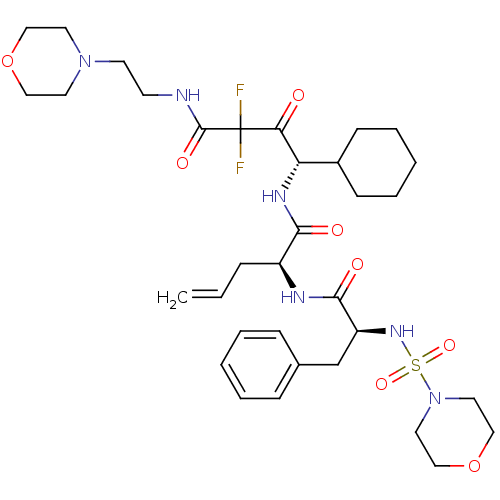 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008158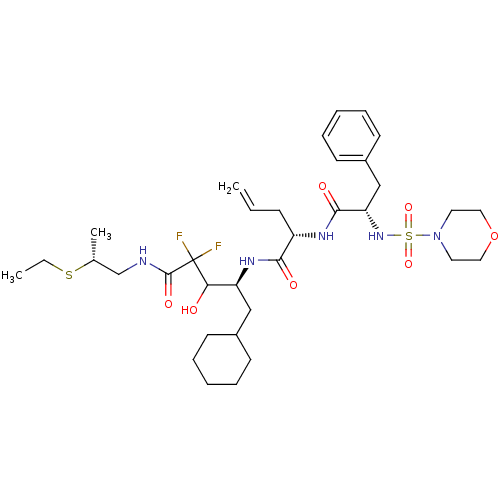 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008133 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008127 (2-[3-Phenyl-2-(piperazine-1-sulfonylamino)-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008136 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844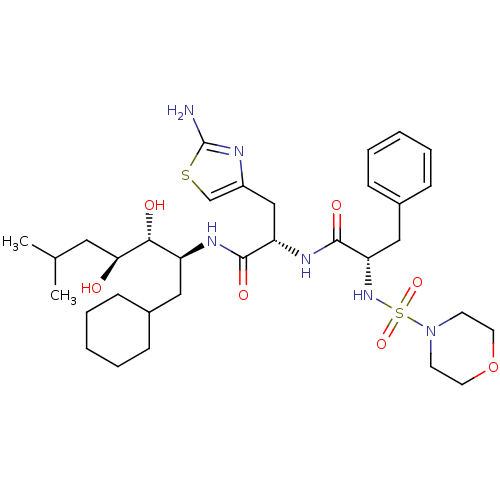 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008122 (5-Cyclohexyl-2,2-difluoro-4-{2-[2-(morpholine-4-su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008137 (2-[2-(4-Methyl-piperazine-1-sulfonylamino)-3-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50283014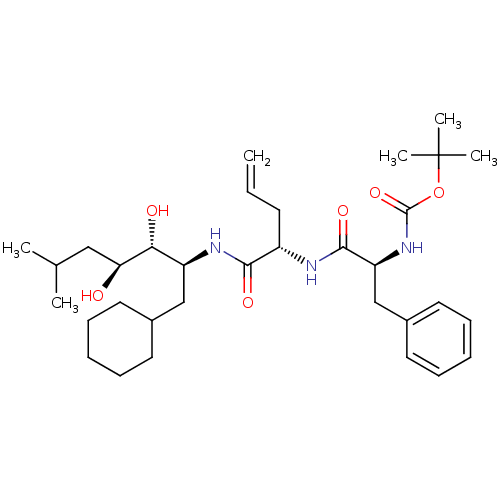 (CHEMBL291139 | {(S)-1-[(S)-1-((1S,2R,3S)-1-Cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008148 (5-Cyclohexyl-2,2-difluoro-3-hydroxy-4-{2-[2-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008142 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006856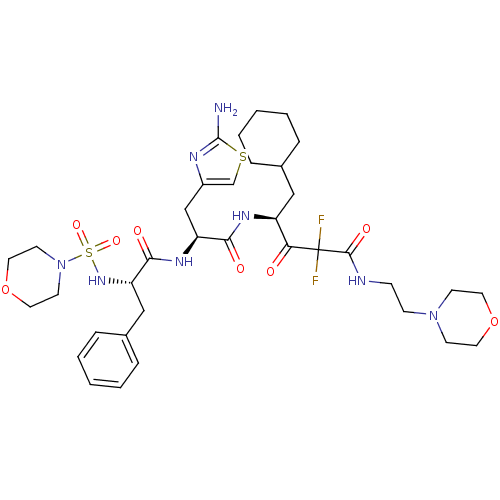 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008154 (2-[3-Phenyl-2-(piperazine-1-sulfonylamino)-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008125 (CHEMBL285207 | N-[1-Cyclohexylmethyl-3,3-difluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008144 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008156 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008162 (CHEMBL31621 | N-[1-Cyclohexylmethyl-3,3-difluoro-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008149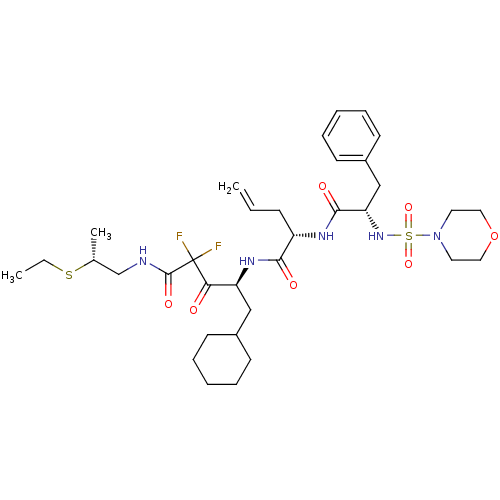 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368310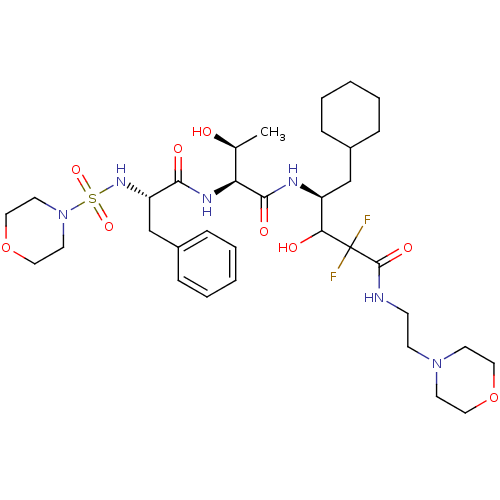 (CHEMBL1790136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008124 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008123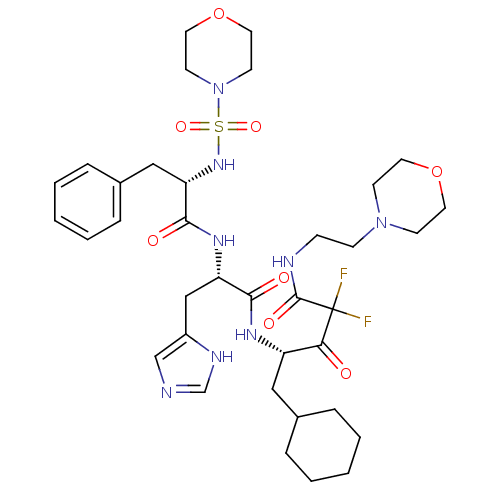 (5-Cyclohexyl-2,2-difluoro-4-{3-(1H-imidazol-4-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008131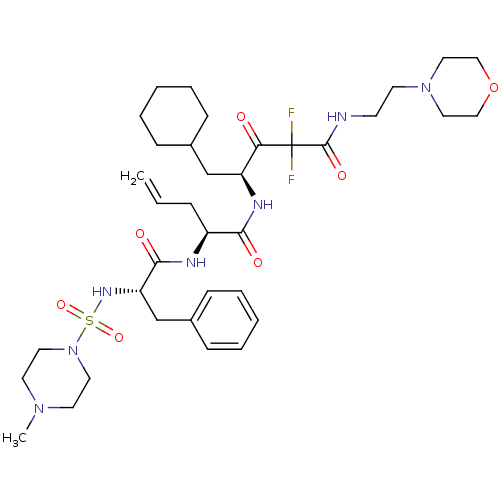 (2-[2-(4-Methyl-piperazine-1-sulfonylamino)-3-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008140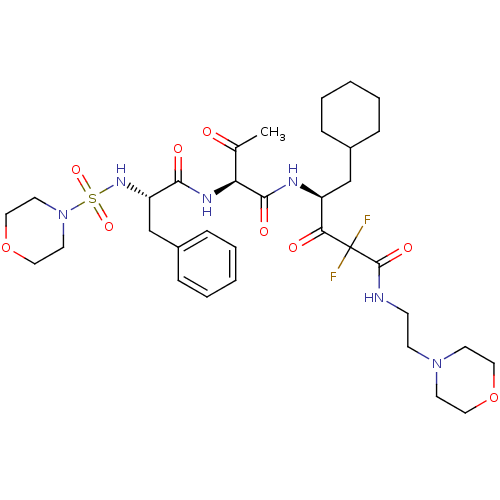 (5-Cyclohexyl-2,2-difluoro-4-{2-[2-(morpholine-4-su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008139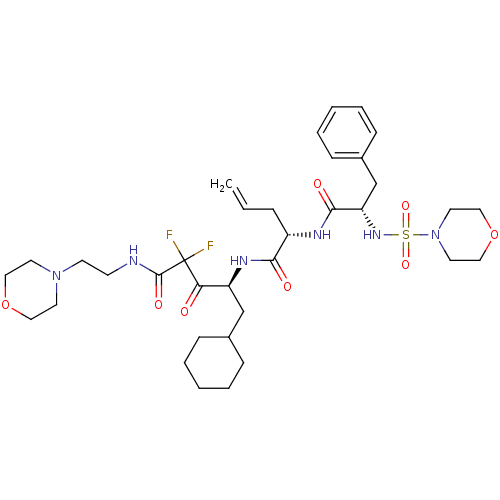 (2-[2-(Morpholine-4-sulfonylamino)-3-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008146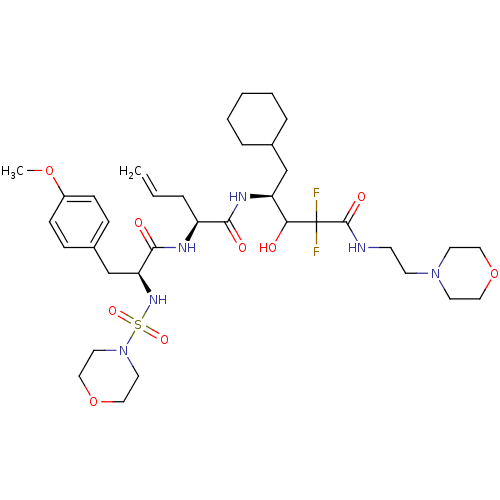 (2-[3-(4-Methoxy-phenyl)-2-(morpholine-4-sulfonylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008157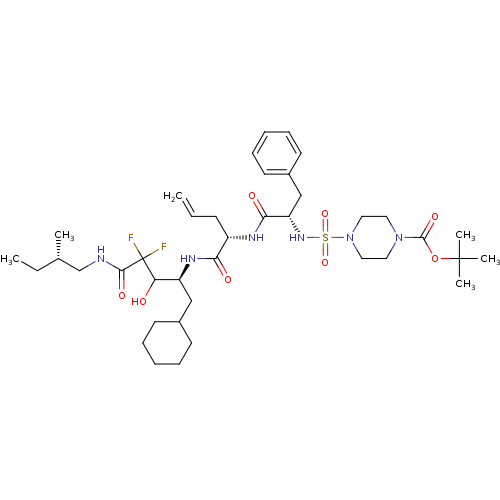 (4-(1-{1-[1-Cyclohexylmethyl-3,3-difluoro-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008161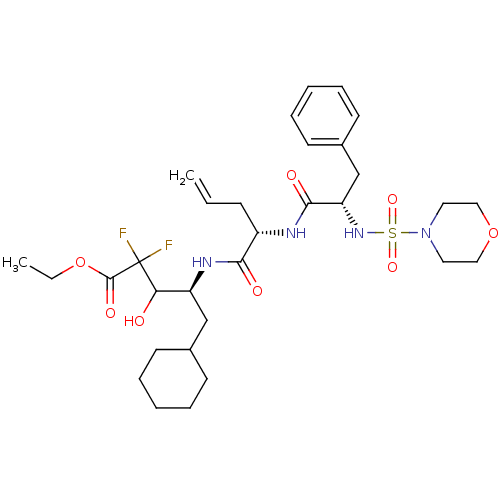 (5-Cyclohexyl-2,2-difluoro-3-hydroxy-4-{2-[2-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008134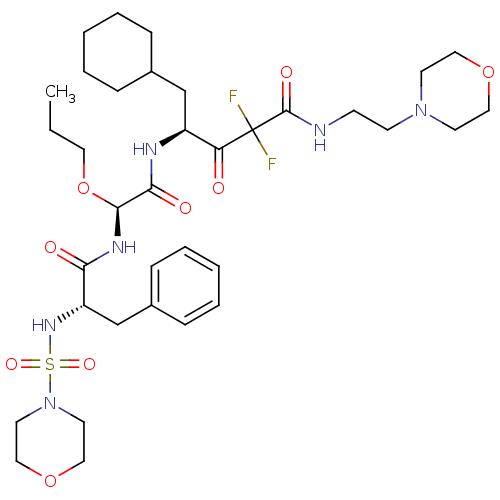 (5-Cyclohexyl-2,2-difluoro-4-{2-[2-(morpholine-4-su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008128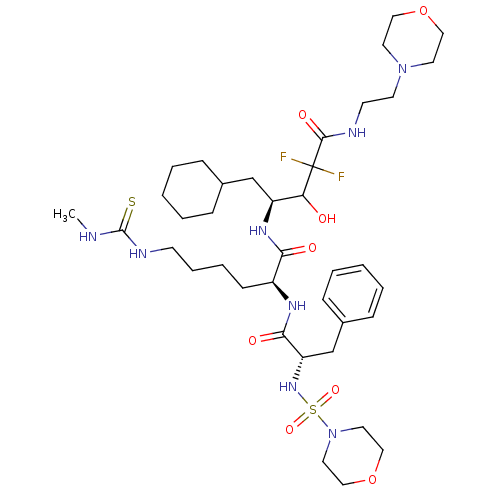 (6-(3-Methyl-thioureido)-2-[2-(morpholine-4-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008152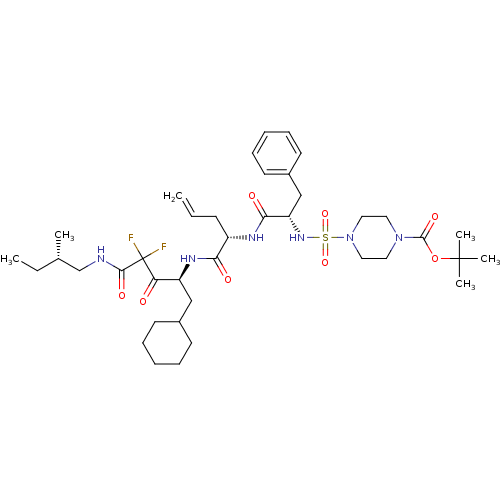 (4-(1-{1-[1-Cyclohexylmethyl-3,3-difluoro-3-(2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006859 (2-((R)-(S)-Morpholine-4-sulfonyl)-1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50283016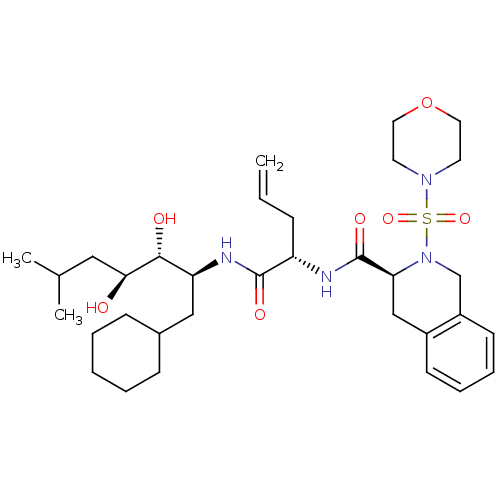 ((S)-2-(Morpholine-4-sulfonyl)-1,2,3,4-tetrahydro-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008126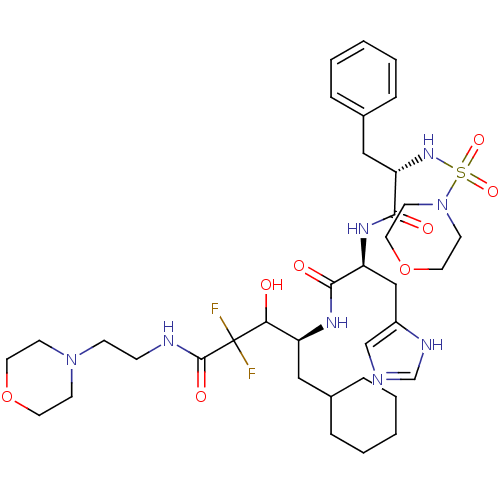 (5-Cyclohexyl-2,2-difluoro-3-hydroxy-4-{3-(1H-imida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50008160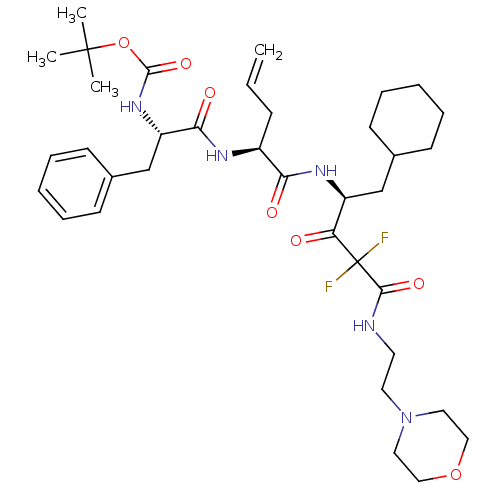 ((1-{1-[1-Cyclohexylmethyl-3,3-difluoro-3-(2-morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The concentration producing 50% inhibition of renin activity in monkey plasma was determined by radioimmunoassay. | J Med Chem 35: 2-14 (1992) BindingDB Entry DOI: 10.7270/Q22J6CGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50283019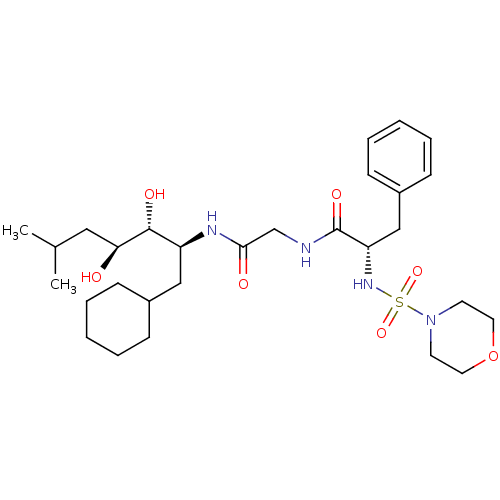 ((S)-N-[((1S,2R,3S)-1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3451 (3-tert-butyl-1-[6-(2,6-dichlorophenyl)-2-{[4-(diet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3453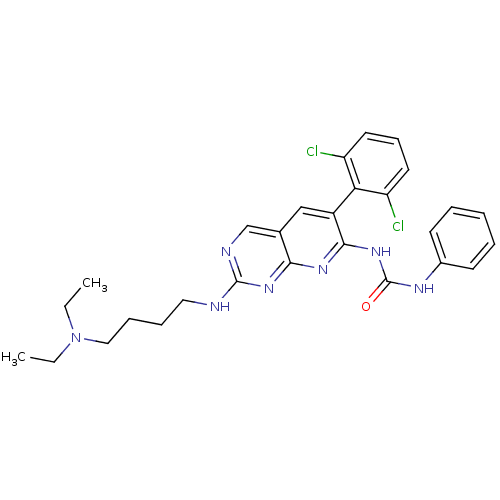 (3-[6-(2,6-dichlorophenyl)-2-{[4-(diethylamino)buty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 578 total ) | Next | Last >> |