

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thioredoxin reductase (Plasmodium falciparum (isolate 3D7)) | BDBM50182122 (CHEMBL204688 | bis(2,4-dinitrophenyl)sulfane) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to Plasmodium falciparum TrxR in presence of thioredoxin | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Plasmodium falciparum (isolate 3D7)) | BDBM50182122 (CHEMBL204688 | bis(2,4-dinitrophenyl)sulfane) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to Plasmodium falciparum TrxR in presence of NADPH | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Plasmodium falciparum (isolate 3D7)) | BDBM50182128 (4-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL383084) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to Plasmodium falciparum TrxR in presence of thioredoxin | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Plasmodium falciparum (isolate 3D7)) | BDBM50182128 (4-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL383084) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to Plasmodium falciparum TrxR in presence of NADPH | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50176071 (2-(2-Amino-ethylamino)-N-(7-{2-[N'-(5-nitro-furan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase | J Med Chem 48: 7024-39 (2005) Article DOI: 10.1021/jm050256l BindingDB Entry DOI: 10.7270/Q2CN73F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50121447 (5-Nitro-furan-2-carboxylic acid N'-(2-naphthalen-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase | J Med Chem 48: 7024-39 (2005) Article DOI: 10.1021/jm050256l BindingDB Entry DOI: 10.7270/Q2CN73F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895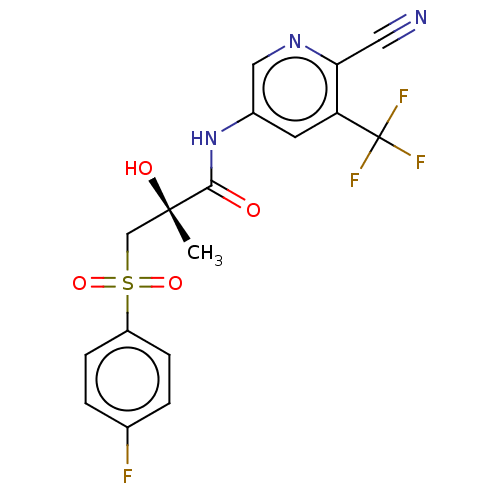 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239191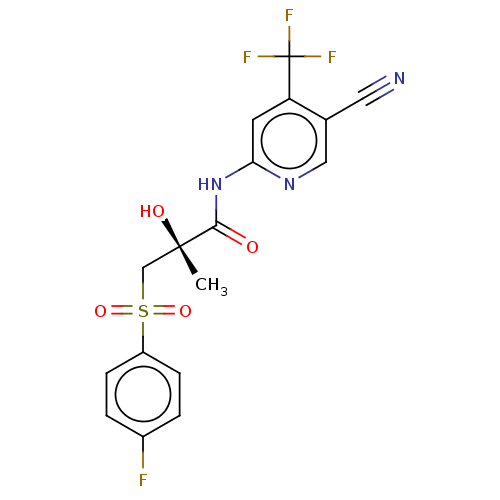 (US10053433, FC 3.077) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of Plasmodium falciparum Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Plasmodium falciparum (isolate 3D7)) | BDBM50182122 (CHEMBL204688 | bis(2,4-dinitrophenyl)sulfane) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum TrxR | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242618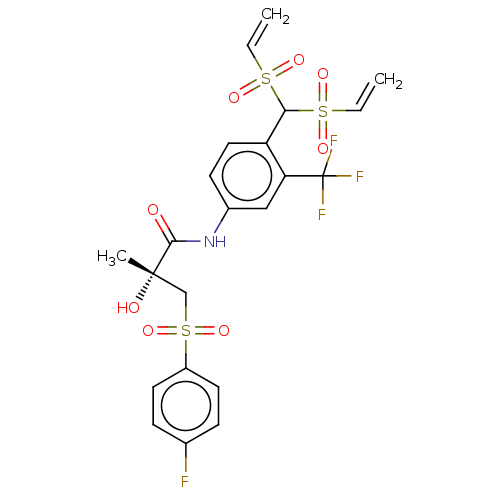 (US10053433, FC 4.025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096035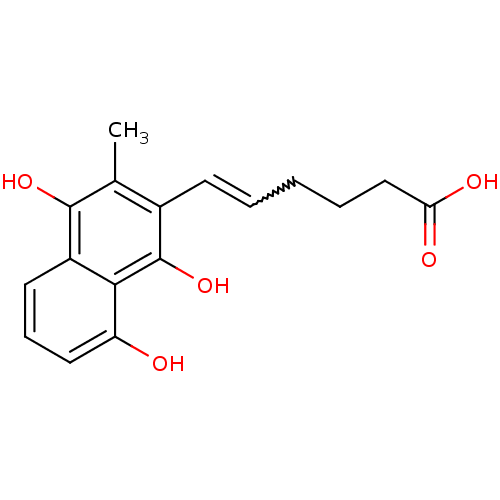 (6-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of human Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242588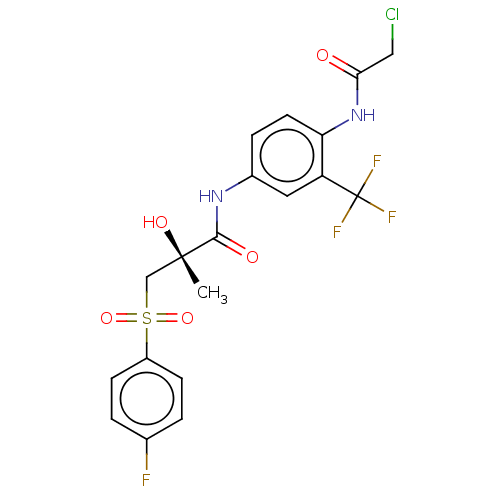 (US10053433, FC 4.039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 750 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396489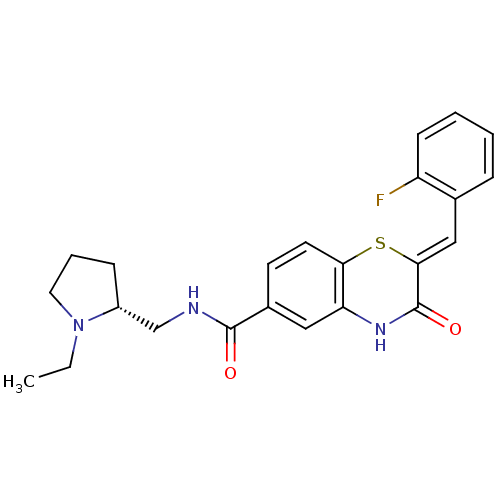 (CHEMBL2170943) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096062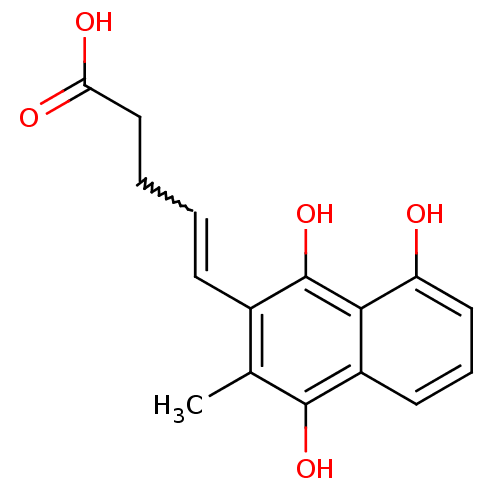 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of human Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242618 (US10053433, FC 4.025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096035 (6-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of Plasmodium falciparum Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396486 (CHEMBL2171113) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396521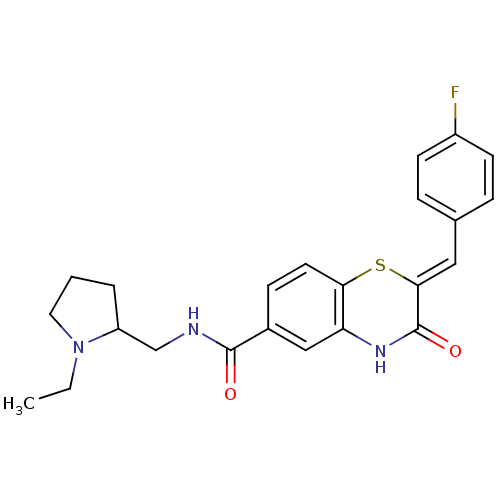 (CHEMBL2170936) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239363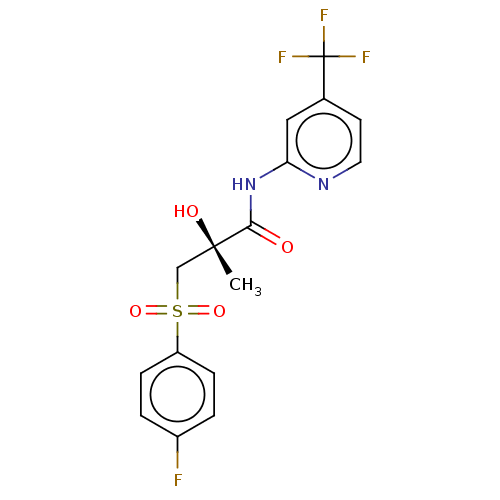 (US10053433, FC 4.126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396519 (CHEMBL2170938) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396486 (CHEMBL2171113) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396489 (CHEMBL2170943) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50425732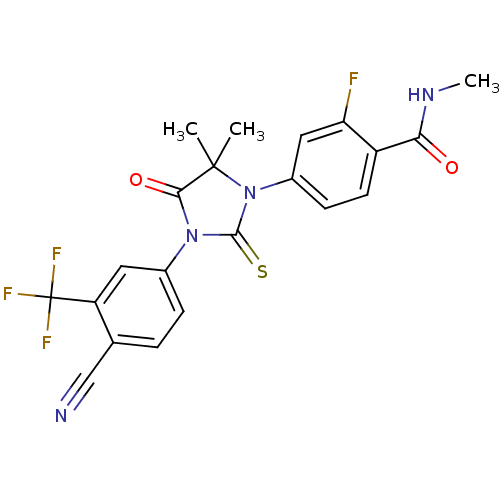 (ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank US Patent | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Plasmodium falciparum (isolate 3D7)) | BDBM50182128 (4-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL383084) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum TrxR | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Plasmodium falciparum (isolate 3D7)) | BDBM50182124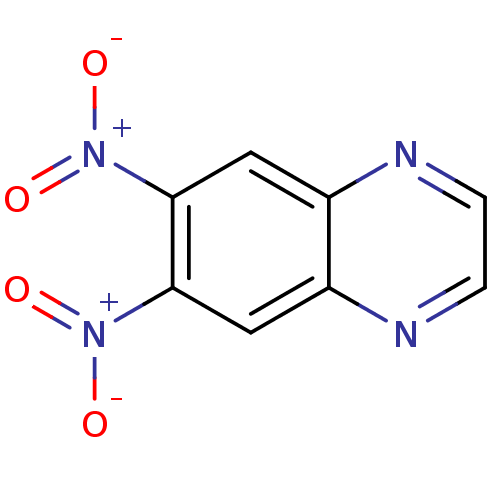 (6,7-dinitroquinoxaline | CHEMBL380953) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum TrxR | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3 (Homo sapiens (Human)) | BDBM50182130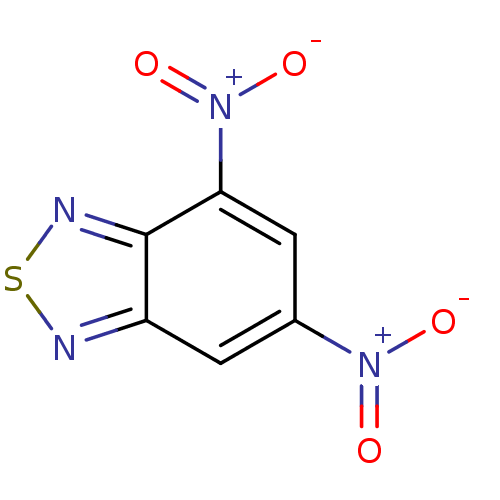 (4,6-dinitrobenzo[c][1,2,5]thiadiazole | CHEMBL2061...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human TrxR | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396521 (CHEMBL2170936) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396522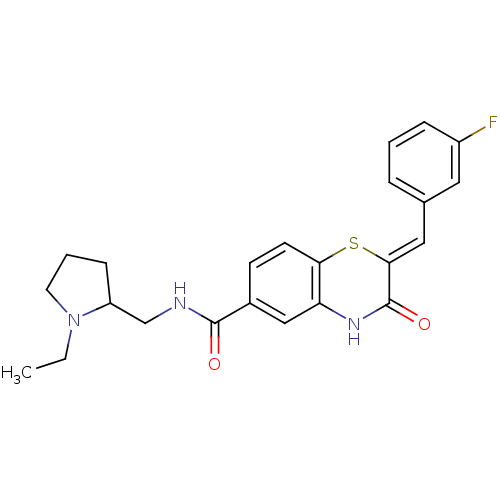 (CHEMBL2170935) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396517 (CHEMBL2170940) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096062 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of Plasmodium falciparum Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239316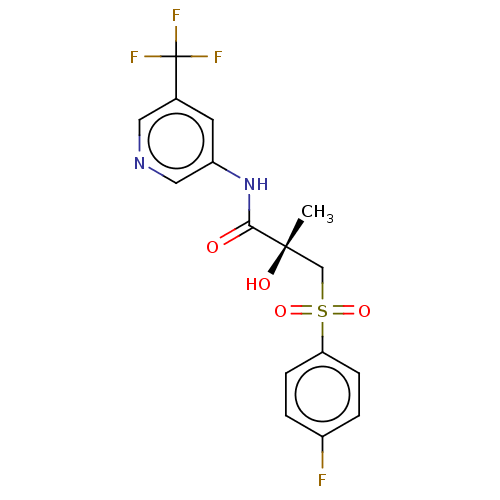 (US10053433, FC 4.116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396488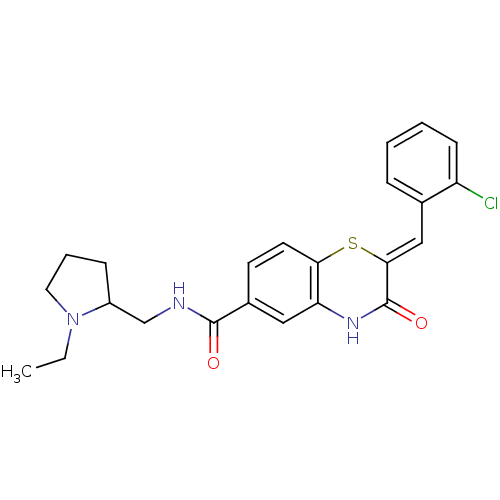 (CHEMBL2170929) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of human Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396519 (CHEMBL2170938) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096071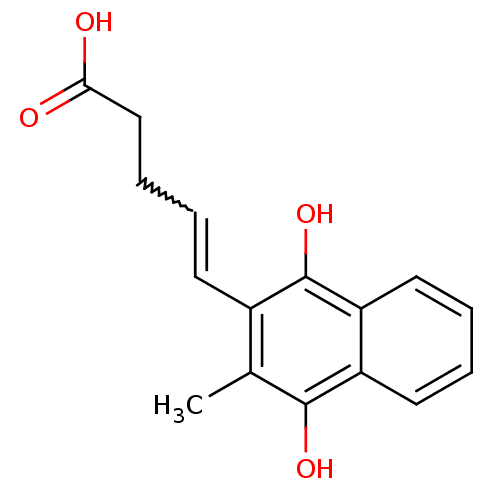 (5-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of human Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396517 (CHEMBL2170940) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396515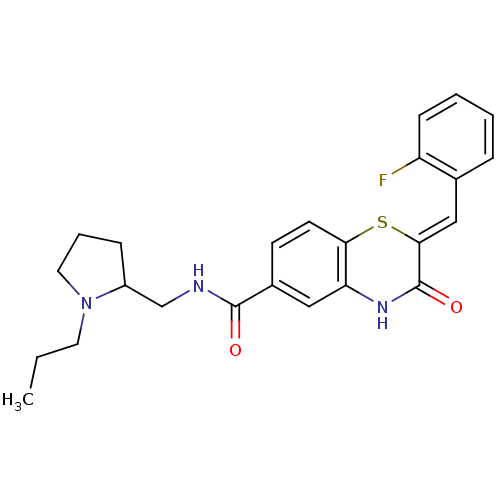 (CHEMBL2170945) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 2 hrs by resazurin/diaphorasecoupled assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50094975 (956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50094975 (956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396488 (CHEMBL2170929) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396520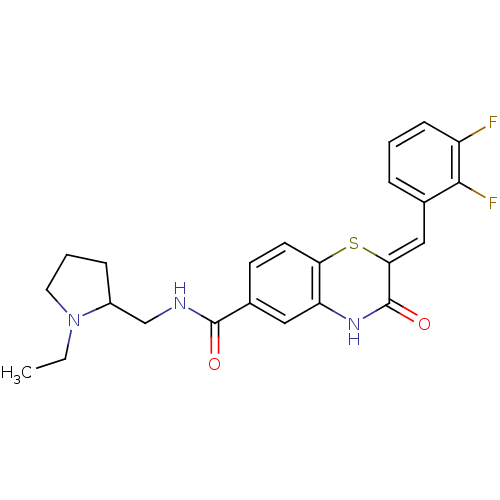 (CHEMBL2170937) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3 (Homo sapiens (Human)) | BDBM50182122 (CHEMBL204688 | bis(2,4-dinitrophenyl)sulfane) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human TrxR | Bioorg Med Chem Lett 16: 2283-92 (2006) Article DOI: 10.1016/j.bmcl.2006.01.027 BindingDB Entry DOI: 10.7270/Q2FB52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096071 (5-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of Plasmodium falciparum Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396515 (CHEMBL2170945) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396522 (CHEMBL2170935) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase (Plasmodium falciparum) | BDBM50396518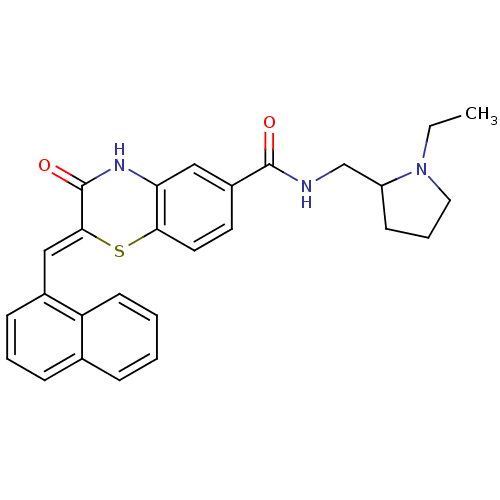 (CHEMBL2170939) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum glucose-6-phosphate dehydrogenase after 45 mins by orthogonal assay | J Med Chem 55: 7262-72 (2012) Article DOI: 10.1021/jm300833h BindingDB Entry DOI: 10.7270/Q25T3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 226 total ) | Next | Last >> |