

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50370447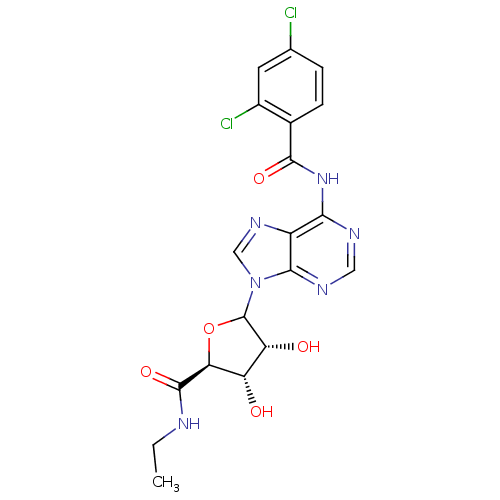 (CHEMBL609341) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station Curated by ChEMBL | Assay Description Inhibition of [3H]R-PIA binding to Adenosine A1 receptor from rat brain membrane | Bioorg Med Chem Lett 15: 2641-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.028 BindingDB Entry DOI: 10.7270/Q2Z60PTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50179030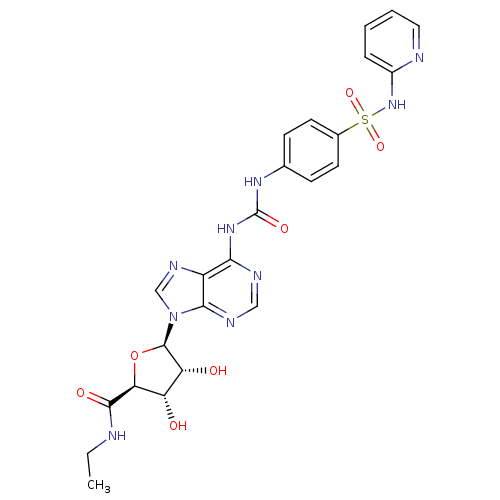 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-(6-{3-[4-(pyridin-2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station"Villa Clara-Cienfuegos" Curated by ChEMBL | Assay Description Displacement of radioligand [125I]AB-MECA binding at rat A3 receptor expressed in CHO cells | Bioorg Med Chem Lett 15: 3491-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.122 BindingDB Entry DOI: 10.7270/Q24Q7VRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50179047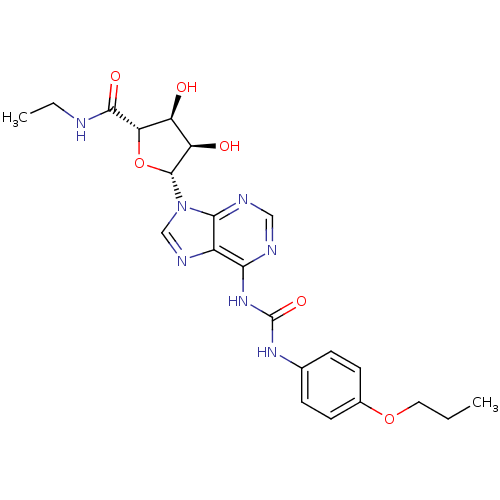 (1-(9-((2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station"Villa Clara-Cienfuegos" Curated by ChEMBL | Assay Description Displacement of radioligand [125I]AB-MECA binding at rat A3 receptor expressed in CHO cells | Bioorg Med Chem Lett 15: 3491-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.122 BindingDB Entry DOI: 10.7270/Q24Q7VRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50370448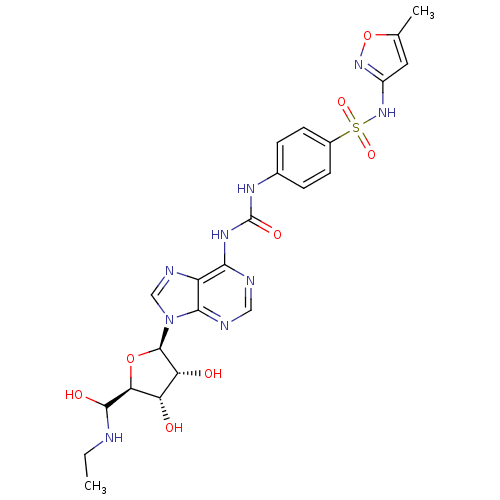 (CHEMBL1791443) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station"Villa Clara-Cienfuegos" Curated by ChEMBL | Assay Description Displacement of radioligand [125I]AB-MECA binding at rat A3 receptor expressed in CHO cells | Bioorg Med Chem Lett 15: 3491-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.122 BindingDB Entry DOI: 10.7270/Q24Q7VRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50179047 (1-(9-((2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station Curated by ChEMBL | Assay Description Inhibition of [3H]R-PIA binding to Adenosine A1 receptor from rat brain membrane | Bioorg Med Chem Lett 15: 2641-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.028 BindingDB Entry DOI: 10.7270/Q2Z60PTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50179030 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-(6-{3-[4-(pyridin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 292 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station Curated by ChEMBL | Assay Description Inhibition of [3H]R-PIA binding to Adenosine A1 receptor from rat brain membrane | Bioorg Med Chem Lett 15: 2641-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.028 BindingDB Entry DOI: 10.7270/Q2Z60PTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50370449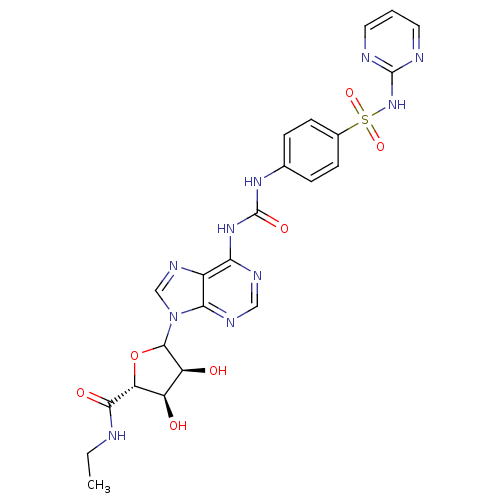 (CHEMBL609340) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station"Villa Clara-Cienfuegos" Curated by ChEMBL | Assay Description Displacement of radioligand [125I]AB-MECA binding at rat A3 receptor expressed in CHO cells | Bioorg Med Chem Lett 15: 3491-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.122 BindingDB Entry DOI: 10.7270/Q24Q7VRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50370449 (CHEMBL609340) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 725 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station Curated by ChEMBL | Assay Description Inhibition of [3H]R-PIA binding to Adenosine A1 receptor from rat brain membrane | Bioorg Med Chem Lett 15: 2641-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.028 BindingDB Entry DOI: 10.7270/Q2Z60PTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50370448 (CHEMBL1791443) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 901 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station Curated by ChEMBL | Assay Description Inhibition of [3H]R-PIA binding to Adenosine A1 receptor from rat brain membrane | Bioorg Med Chem Lett 15: 2641-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.028 BindingDB Entry DOI: 10.7270/Q2Z60PTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50372286 (CHEMBL257111) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas Curated by ChEMBL | Assay Description Displacement of [3H]R-N6-phenylisopropyladenosine from adenosine A1 receptor in rat cerebral cortical membrane | Bioorg Med Chem 16: 1658-75 (2008) Article DOI: 10.1016/j.bmc.2007.11.026 BindingDB Entry DOI: 10.7270/Q20C4WMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50372288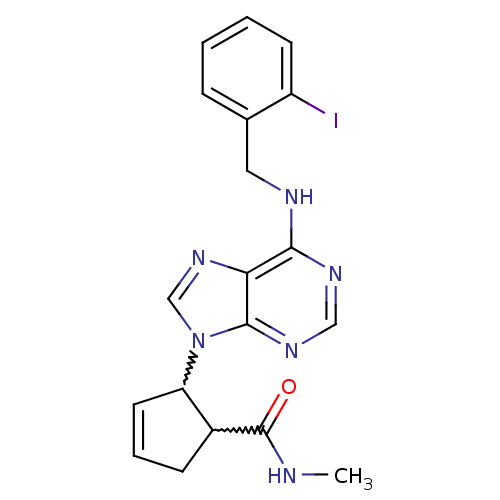 (CHEMBL272016) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas Curated by ChEMBL | Assay Description Displacement of [3H]R-N6-phenylisopropyladenosine from adenosine A1 receptor in rat cerebral cortical membrane | Bioorg Med Chem 16: 1658-75 (2008) Article DOI: 10.1016/j.bmc.2007.11.026 BindingDB Entry DOI: 10.7270/Q20C4WMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50370450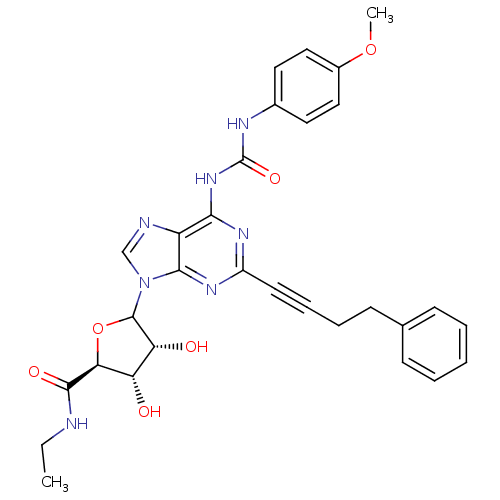 (CHEMBL610601) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Sugar Cane Station Curated by ChEMBL | Assay Description Inhibition of [3H]R-PIA binding to Adenosine A1 receptor from rat brain membrane | Bioorg Med Chem Lett 15: 2641-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.028 BindingDB Entry DOI: 10.7270/Q2Z60PTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50372285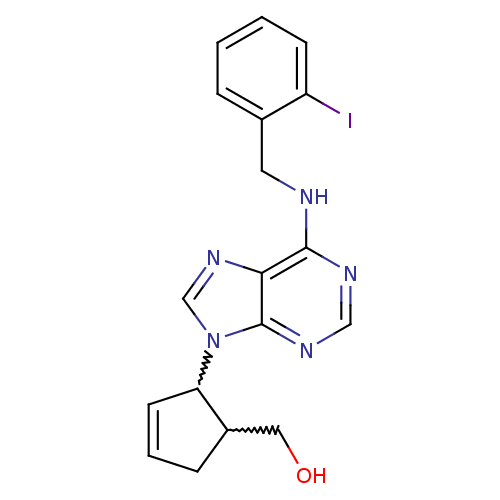 (CHEMBL257684) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas Curated by ChEMBL | Assay Description Displacement of [3H]R-N6-phenylisopropyladenosine from adenosine A1 receptor in rat cerebral cortical membrane | Bioorg Med Chem 16: 1658-75 (2008) Article DOI: 10.1016/j.bmc.2007.11.026 BindingDB Entry DOI: 10.7270/Q20C4WMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50372287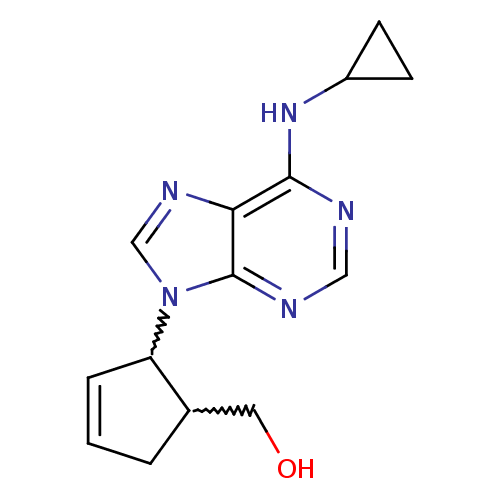 (CHEMBL405897) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas Curated by ChEMBL | Assay Description Displacement of [3H]R-N6-phenylisopropyladenosine from adenosine A1 receptor in rat cerebral cortical membrane | Bioorg Med Chem 16: 1658-75 (2008) Article DOI: 10.1016/j.bmc.2007.11.026 BindingDB Entry DOI: 10.7270/Q20C4WMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456804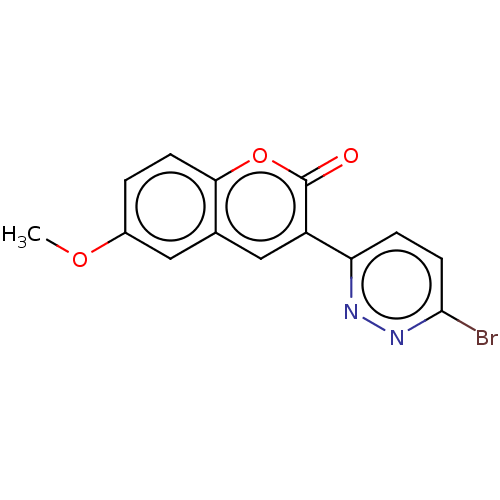 (CHEMBL4215743) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456815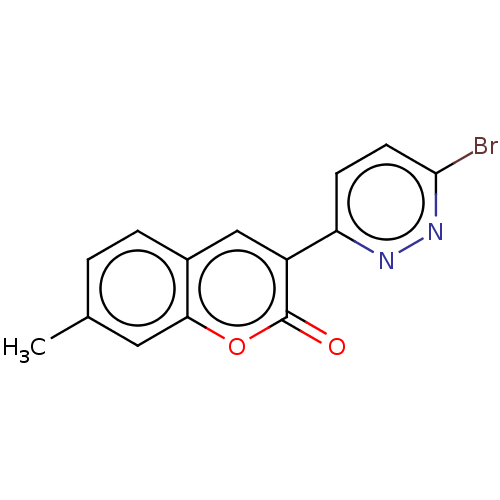 (CHEMBL4203878) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456802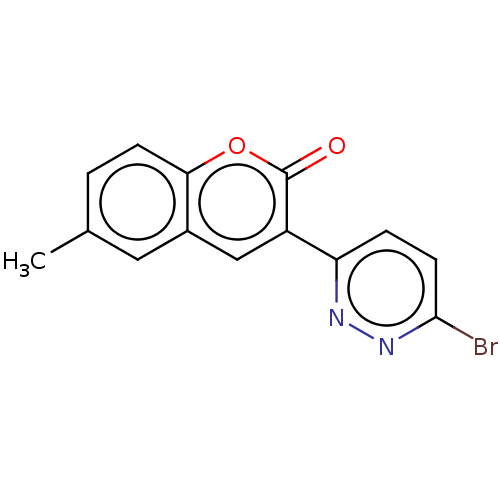 (CHEMBL4208757) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456811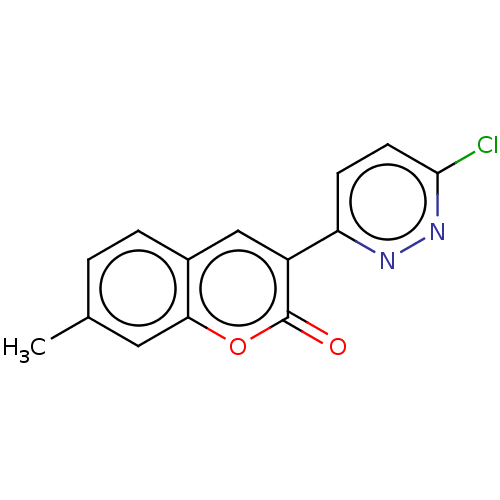 (CHEMBL4215220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456801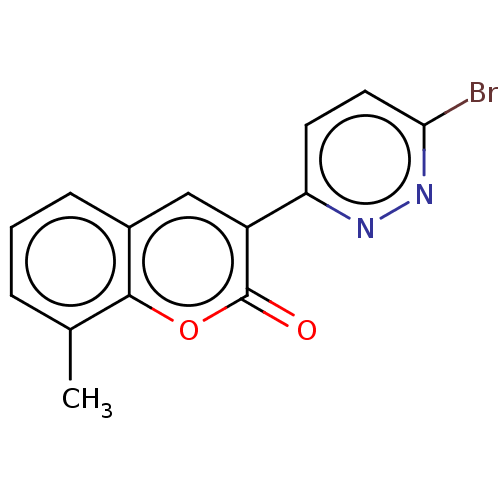 (CHEMBL4202487) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456812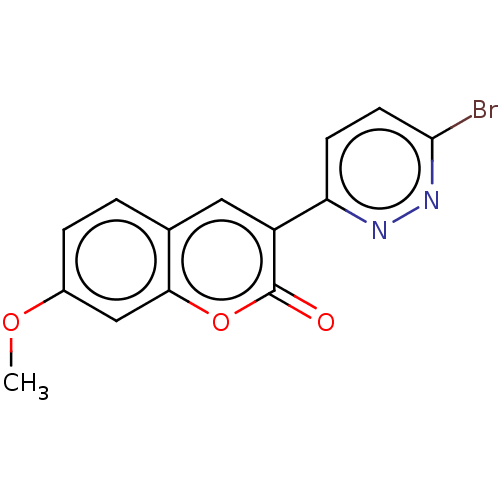 (CHEMBL4214681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456803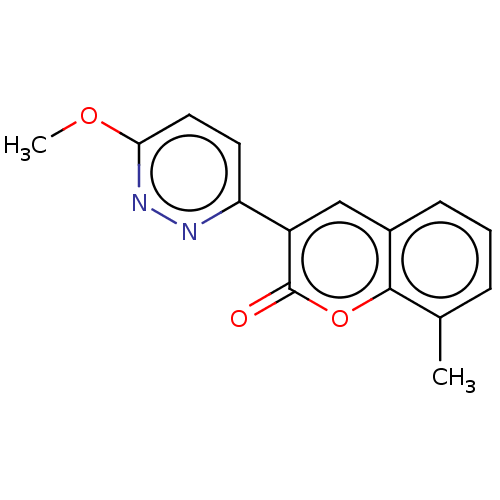 (CHEMBL4206334) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM29136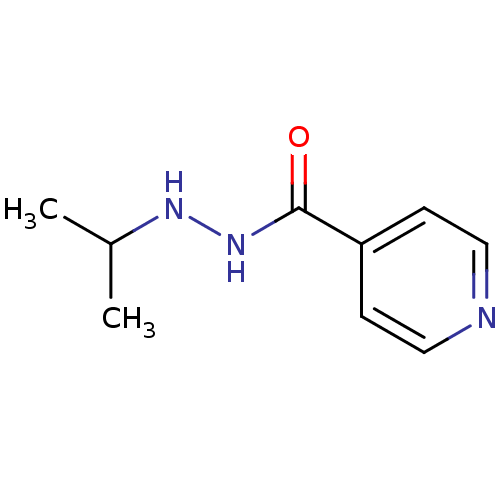 (CHEMBL92401 | Euphozid | Iprazid | Iproniazid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM29136 (CHEMBL92401 | Euphozid | Iprazid | Iproniazid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456807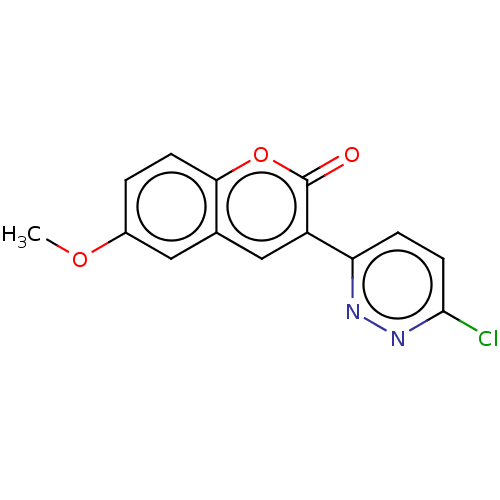 (CHEMBL4210805) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456810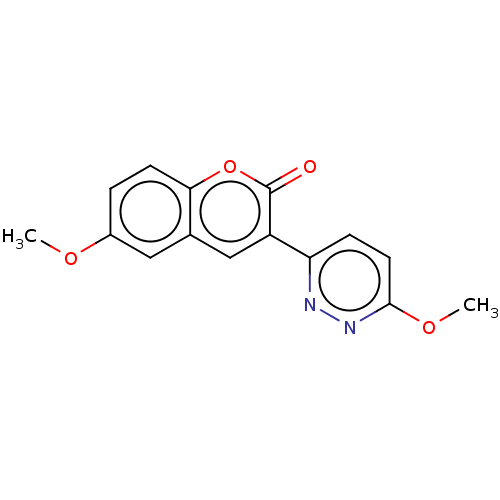 (CHEMBL4218045) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456805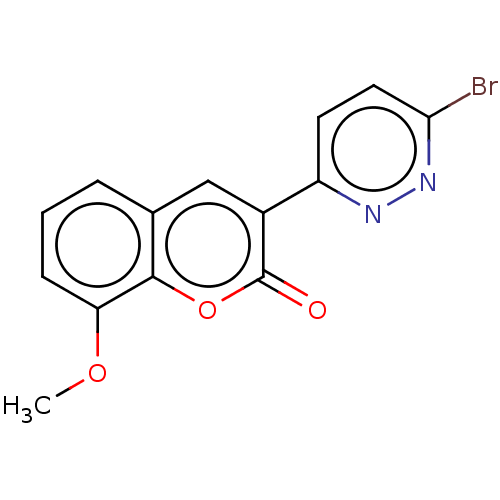 (CHEMBL4206008) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456814 (CHEMBL4205833) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456806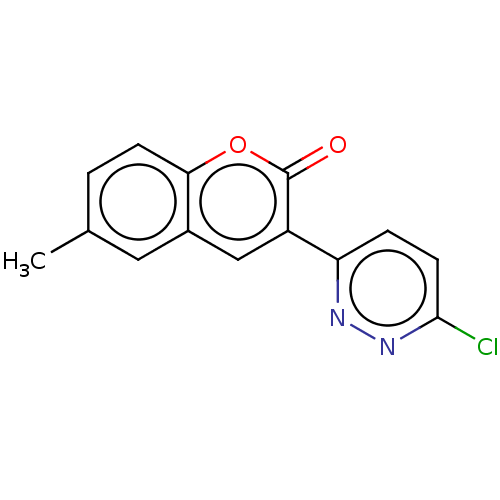 (CHEMBL4210287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456809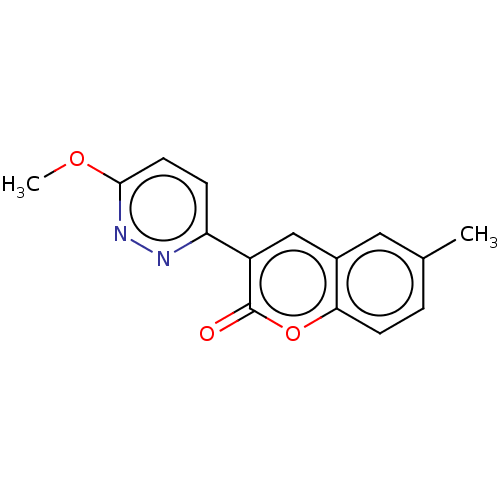 (CHEMBL4202589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456808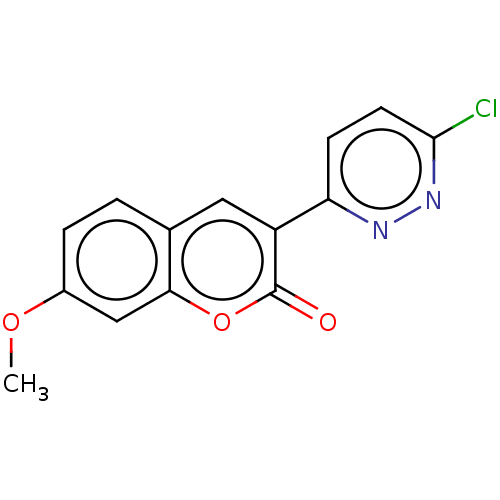 (CHEMBL4218157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456813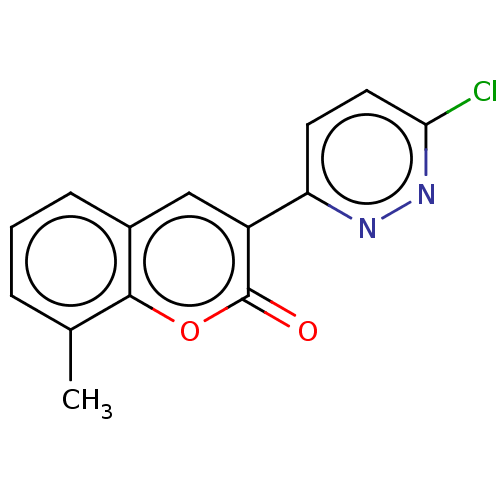 (CHEMBL4205924) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456816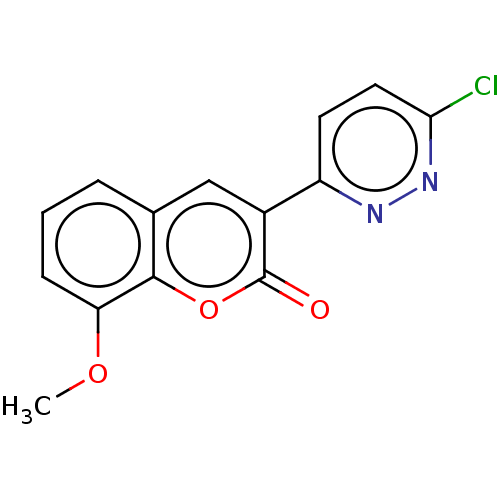 (CHEMBL4206431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A expressed in baculovirus infected BTI-TN5B1-4 cells using p-tyramine as substrate assessed as reduction in H2O2... | Eur J Med Chem 139: 1-11 (2017) Article DOI: 10.1016/j.ejmech.2017.07.045 BindingDB Entry DOI: 10.7270/Q2MC92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194146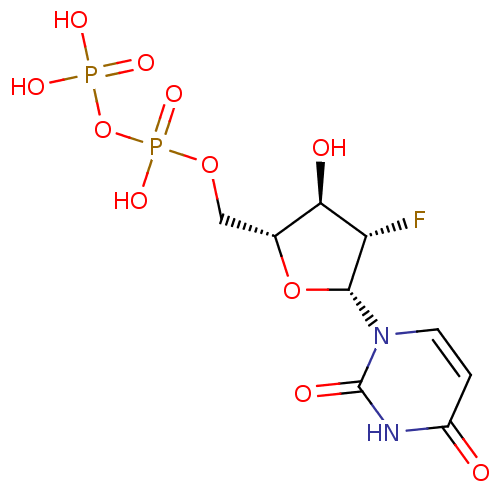 ((2R,3S,4R,5R)-1-(5-(diphosphoryloxymethyl)-3-fluor...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118239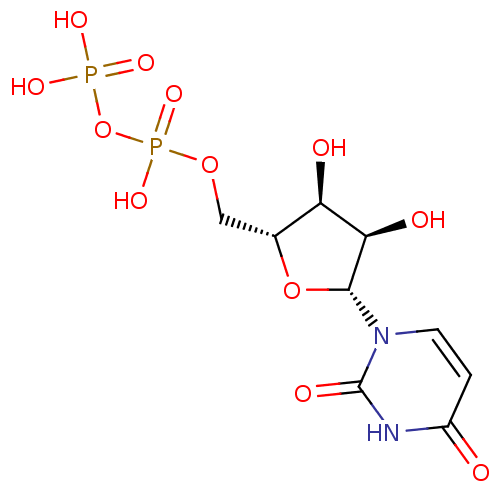 (CHEMBL130266 | UDP | Uridine diphosphate | uridine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194147 (4-Thio-UDP | CHEMBL384992 | [(2R,3S,4R,5R)-3,4-dih...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194148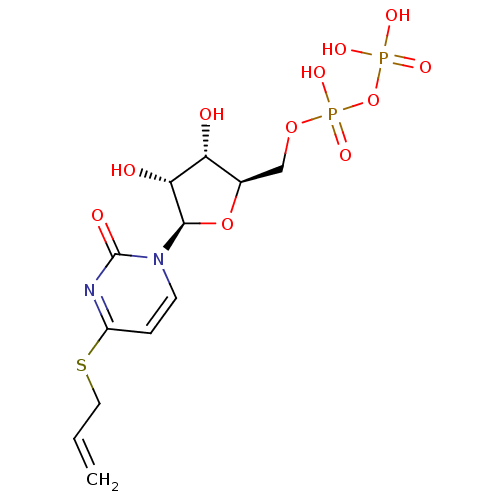 ((2R,3R,4S,5R)-4-(allylthio)-1-(3,4-dihydroxy-5-(di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM92459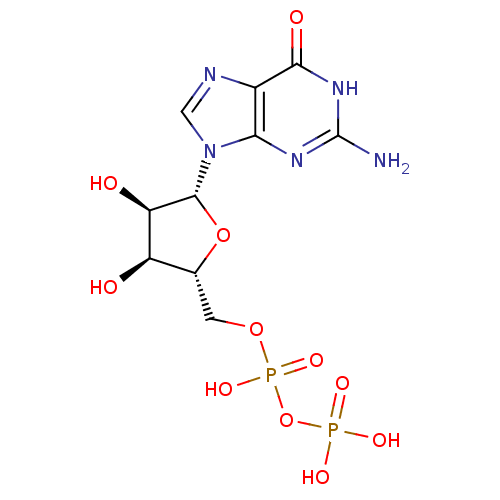 (CHEMBL384759 | GDP | Guanosine Diphosphate) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194150 ((1S,3R,4R,5S)-1-(4-hydroxy-1-(diphosphoryloxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194152 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194153 (5'-CDP | CDP | CHEMBL425252 | Cytidine | Cytidine ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194151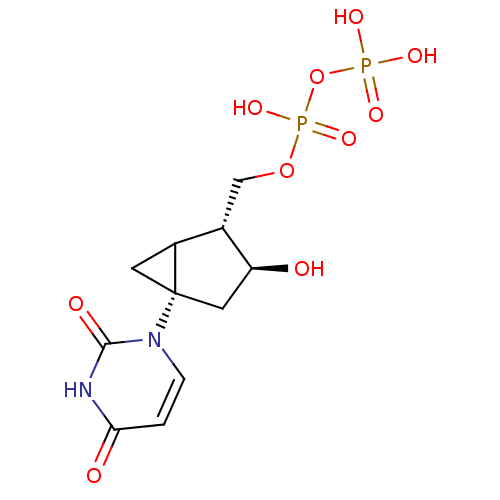 (CHEMBL212090 | [(2R,3S,5S)-5-(2,4-dioxo-3,4-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194154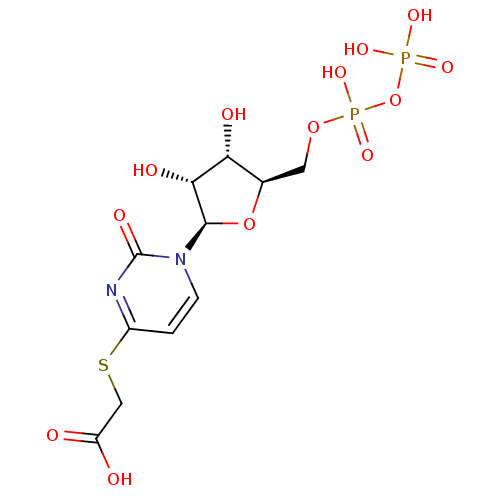 ((2R,3R,4S,5R)-2-(1-(3,4-dihydroxy-5-(diphosphorylo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194155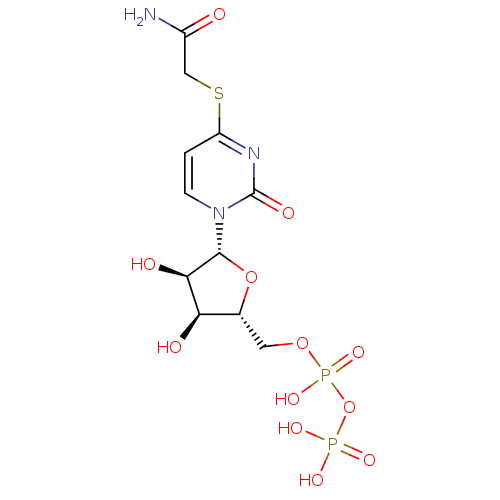 ((2R,3R,4S,5R)-2-(1-(3,4-dihydroxy-5-(diphosphorylo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50179185 (((2R,3S,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194156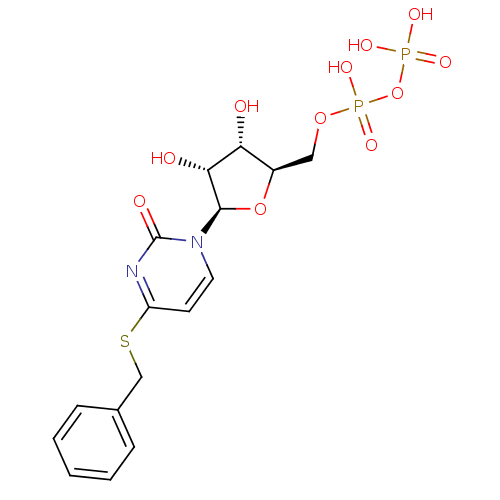 ((2R,3R,4S,5R)-4-(benzylthio)-1-(3,4-dihydroxy-5-(d...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194157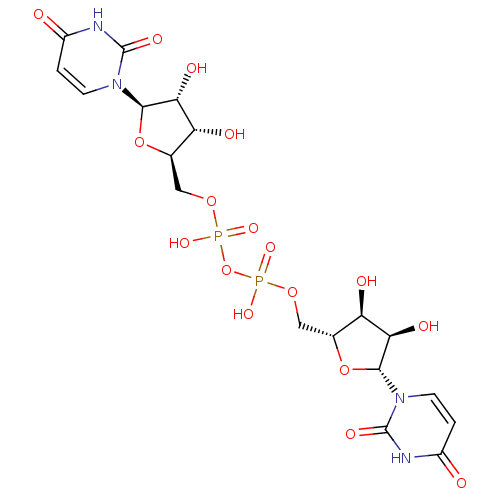 (CHEMBL378445 | {[(2R,3S,4R,5R)-5-(2,4-dioxo-1,2,3,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194158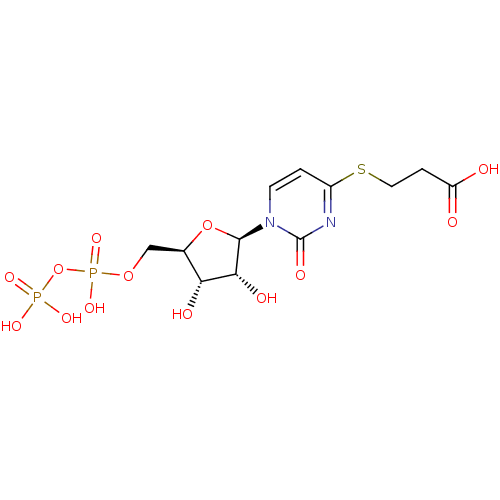 ((2R,3R,4S,5R)-3-(1-(3,4-dihydroxy-5-(diphosphorylo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 194 total ) | Next | Last >> |