 Found 109 hits with Last Name = 'butler' and Initial = 'hs'
Found 109 hits with Last Name = 'butler' and Initial = 'hs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50265896
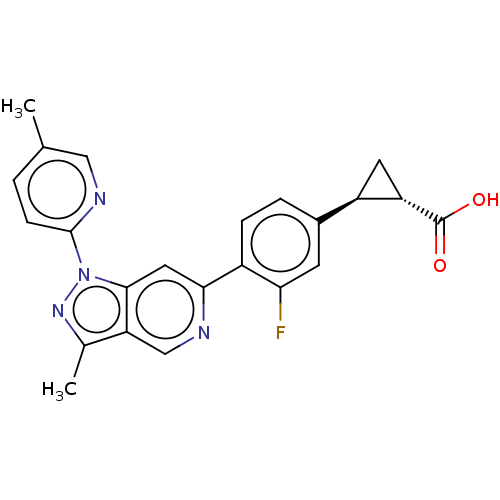
(CHEMBL4070359)Show SMILES Cc1nn(-c2ccc(C)cn2)c2cc(ncc12)-c1ccc(cc1F)[C@H]1C[C@@H]1C(O)=O |r| Show InChI InChI=1S/C23H19FN4O2/c1-12-3-6-22(26-10-12)28-21-9-20(25-11-18(21)13(2)27-28)15-5-4-14(7-19(15)24)16-8-17(16)23(29)30/h3-7,9-11,16-17H,8H2,1-2H3,(H,29,30)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Adenosine A2A receptor (unknown origin) |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600005
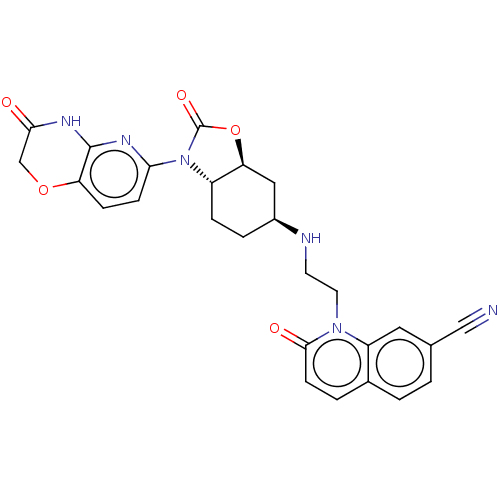
(CHEMBL5195268)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(ccc2ccc1=O)C#N |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600003
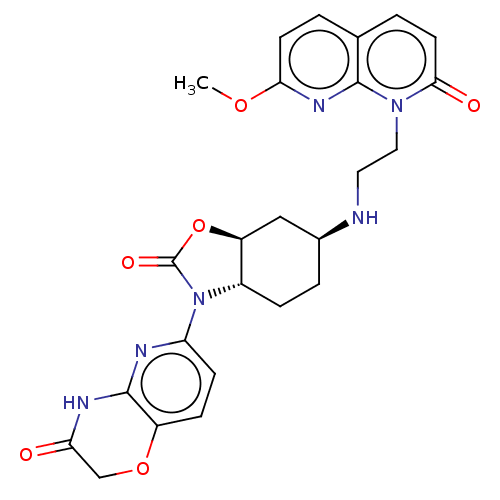
(CHEMBL5201228)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50050549

(GSK2140944 | Gepotidacin)Show SMILES O=c1ccc2ncc(=O)n3[C@H](CN4CCC(CC4)NCc4cc5CCCOc5cn4)Cn1c23 |r| Show InChI InChI=1S/C24H28N6O3/c31-22-4-3-20-24-29(22)15-19(30(24)23(32)13-27-20)14-28-7-5-17(6-8-28)25-11-18-10-16-2-1-9-33-21(16)12-26-18/h3-4,10,12-13,17,19,25H,1-2,5-9,11,14-15H2/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600006

(CHEMBL5172158)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600007
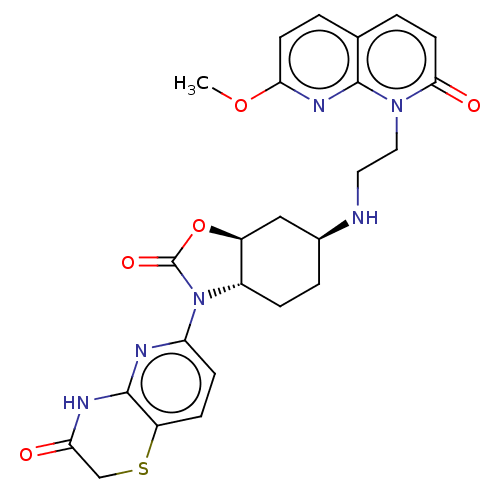
(CHEMBL5182200)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2SCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600004
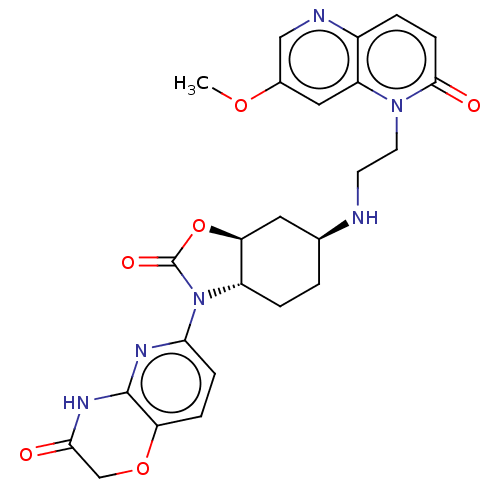
(CHEMBL5184104)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(OC)cnc2ccc1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600009
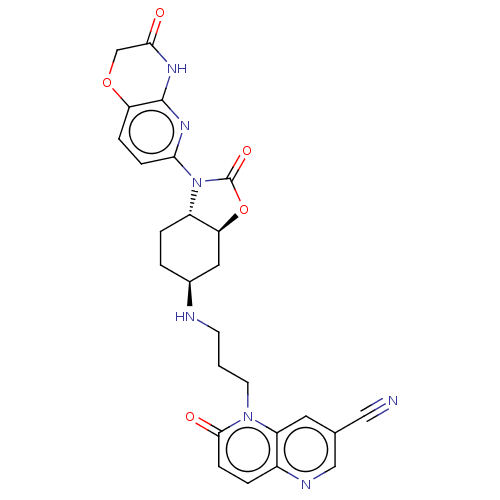
(CHEMBL5177076)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600008
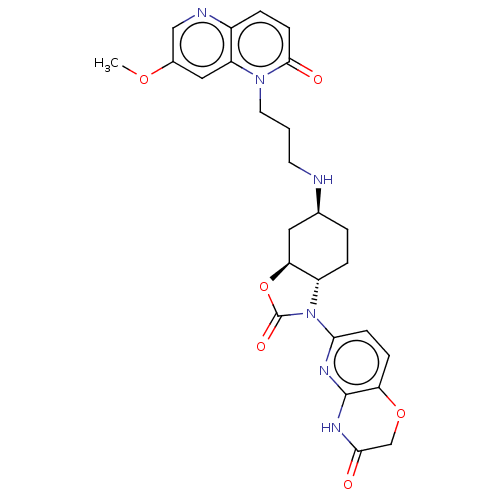
(CHEMBL5198597)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(OC)cnc2ccc1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50265895
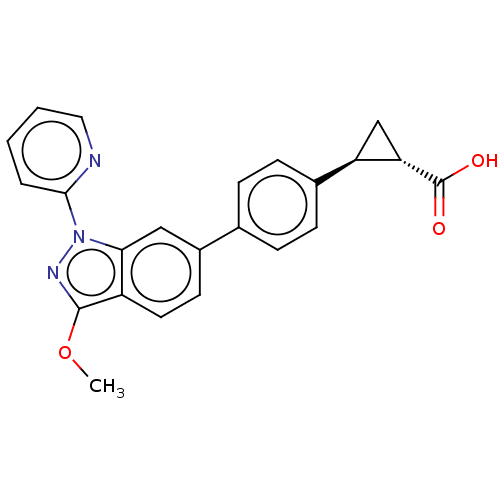
(CHEMBL4097537)Show SMILES COc1nn(-c2ccccn2)c2cc(ccc12)-c1ccc(cc1)[C@H]1C[C@@H]1C(O)=O |r| Show InChI InChI=1S/C23H19N3O3/c1-29-22-17-10-9-16(12-20(17)26(25-22)21-4-2-3-11-24-21)14-5-7-15(8-6-14)18-13-19(18)23(27)28/h2-12,18-19H,13H2,1H3,(H,27,28)/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 4D (unknown origin) |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600011
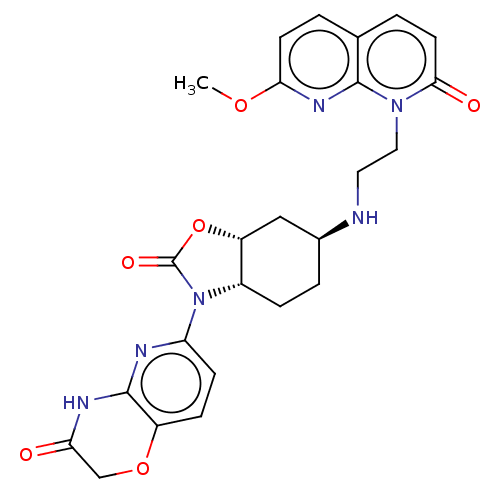
(CHEMBL5205047)Show SMILES [H][C@@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600007

(CHEMBL5182200)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2SCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600010

(CHEMBL5184352)Show SMILES [H][C@]12C[C@@H](CC[C@@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600003

(CHEMBL5201228)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600013
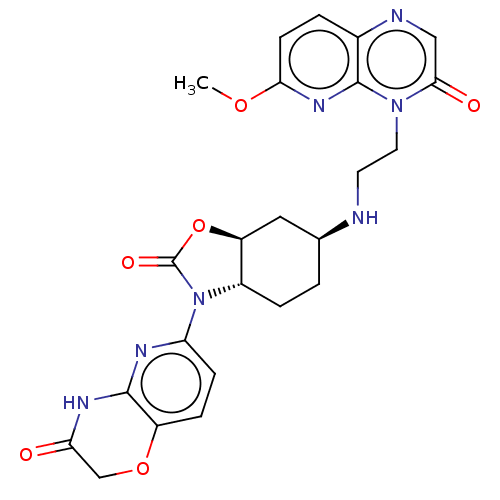
(CHEMBL5194618)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ncc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600018
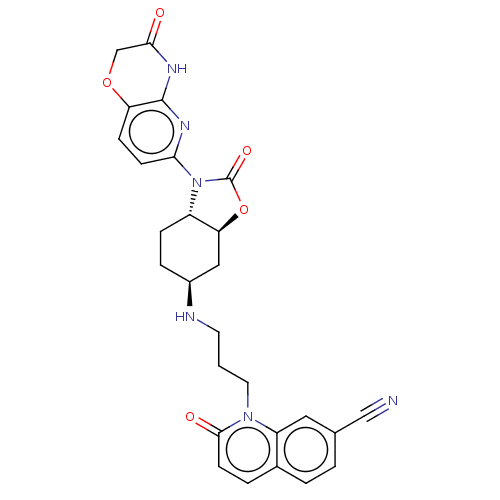
(CHEMBL5199405)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(ccc2ccc1=O)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600014
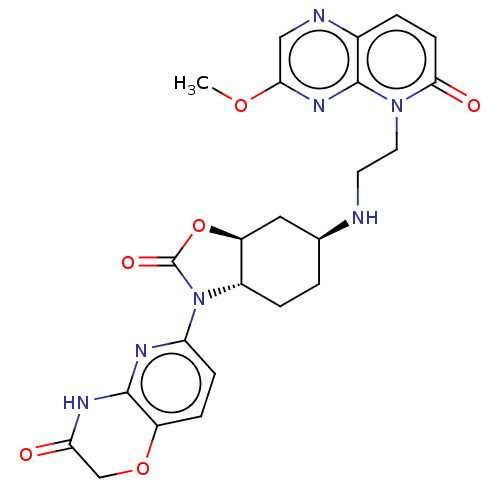
(CHEMBL5172094)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)cnc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600012

(CHEMBL5190832)Show SMILES [H][C@@]12C[C@@H](CC[C@@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600017
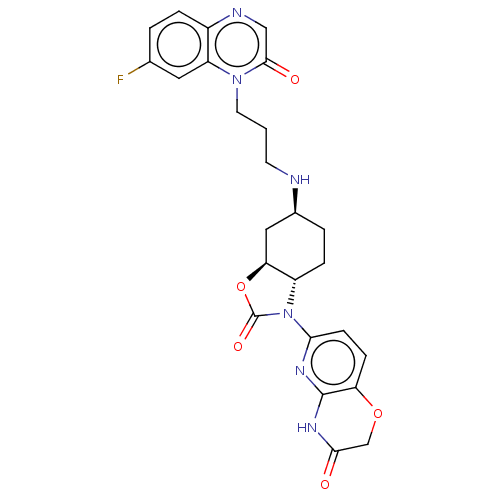
(CHEMBL5195721)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(F)ccc2ncc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50600003

(CHEMBL5201228)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600015
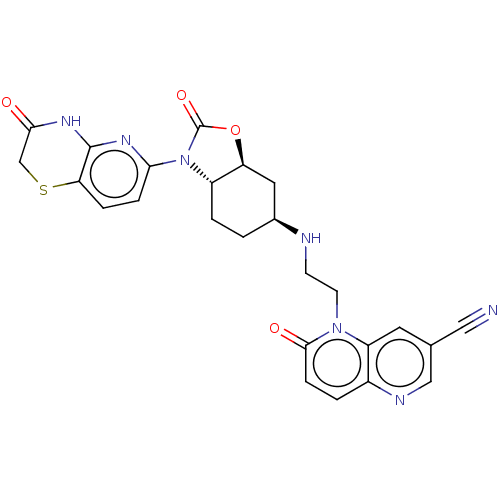
(CHEMBL5206169)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2SCC(=O)Nc2n1)NCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600004

(CHEMBL5184104)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(OC)cnc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600016

(CHEMBL5170129)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ncc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50050549

(GSK2140944 | Gepotidacin)Show SMILES O=c1ccc2ncc(=O)n3[C@H](CN4CCC(CC4)NCc4cc5CCCOc5cn4)Cn1c23 |r| Show InChI InChI=1S/C24H28N6O3/c31-22-4-3-20-24-29(22)15-19(30(24)23(32)13-27-20)14-28-7-5-17(6-8-28)25-11-18-10-16-2-1-9-33-21(16)12-26-18/h3-4,10,12-13,17,19,25H,1-2,5-9,11,14-15H2/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50600005

(CHEMBL5195268)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(ccc2ccc1=O)C#N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50600007

(CHEMBL5182200)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2SCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50050549

(GSK2140944 | Gepotidacin)Show SMILES O=c1ccc2ncc(=O)n3[C@H](CN4CCC(CC4)NCc4cc5CCCOc5cn4)Cn1c23 |r| Show InChI InChI=1S/C24H28N6O3/c31-22-4-3-20-24-29(22)15-19(30(24)23(32)13-27-20)14-28-7-5-17(6-8-28)25-11-18-10-16-2-1-9-33-21(16)12-26-18/h3-4,10,12-13,17,19,25H,1-2,5-9,11,14-15H2/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50600006

(CHEMBL5172158)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50600004

(CHEMBL5184104)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(OC)cnc2ccc1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600009

(CHEMBL5177076)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600008

(CHEMBL5198597)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(OC)cnc2ccc1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50600008

(CHEMBL5198597)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(OC)cnc2ccc1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600006

(CHEMBL5172158)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50600005

(CHEMBL5195268)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(ccc2ccc1=O)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50600009

(CHEMBL5177076)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265860

(CHEMBL4082804)Show SMILES Cc1nn(-c2ccccn2)c2cc(ccc12)-c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C21H17N3O2/c1-14-18-10-9-17(16-7-5-15(6-8-16)12-21(25)26)13-19(18)24(23-14)20-4-2-3-11-22-20/h2-11,13H,12H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.70E+4 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265862
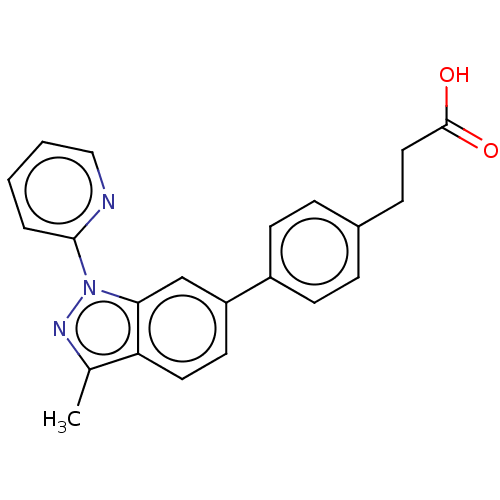
(CHEMBL4077507)Show SMILES Cc1nn(-c2ccccn2)c2cc(ccc12)-c1ccc(CCC(O)=O)cc1 Show InChI InChI=1S/C22H19N3O2/c1-15-19-11-10-18(17-8-5-16(6-9-17)7-12-22(26)27)14-20(19)25(24-15)21-4-2-3-13-23-21/h2-6,8-11,13-14H,7,12H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265865
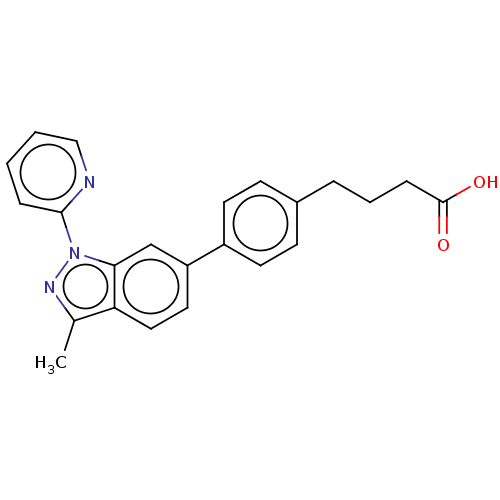
(CHEMBL4076190)Show SMILES Cc1nn(-c2ccccn2)c2cc(ccc12)-c1ccc(CCCC(O)=O)cc1 Show InChI InChI=1S/C23H21N3O2/c1-16-20-13-12-19(15-21(20)26(25-16)22-6-2-3-14-24-22)18-10-8-17(9-11-18)5-4-7-23(27)28/h2-3,6,8-15H,4-5,7H2,1H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265868
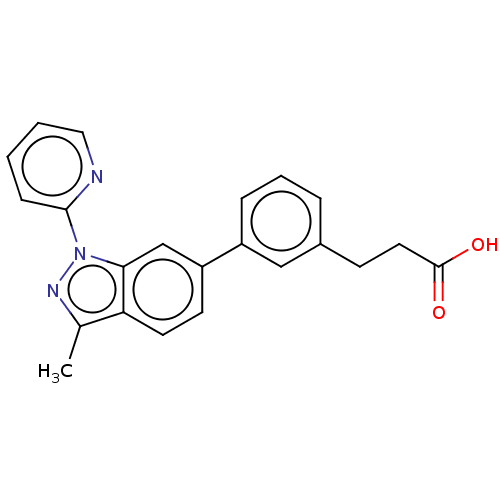
(CHEMBL4071228)Show SMILES Cc1nn(-c2ccccn2)c2cc(ccc12)-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C22H19N3O2/c1-15-19-10-9-18(17-6-4-5-16(13-17)8-11-22(26)27)14-20(19)25(24-15)21-7-2-3-12-23-21/h2-7,9-10,12-14H,8,11H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.70E+4 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265874
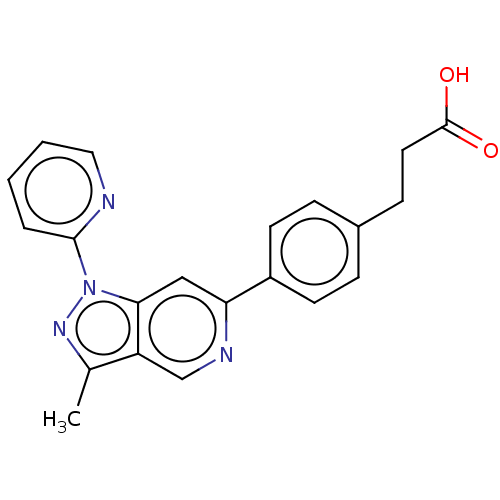
(CHEMBL4085695)Show SMILES Cc1nn(-c2ccccn2)c2cc(ncc12)-c1ccc(CCC(O)=O)cc1 Show InChI InChI=1S/C21H18N4O2/c1-14-17-13-23-18(16-8-5-15(6-9-16)7-10-21(26)27)12-19(17)25(24-14)20-4-2-3-11-22-20/h2-6,8-9,11-13H,7,10H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265875
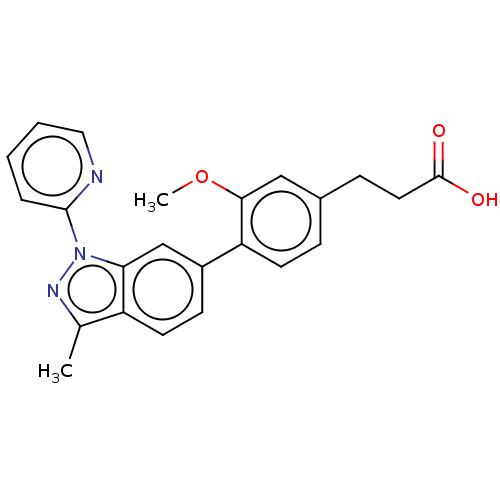
(CHEMBL4070273)Show SMILES COc1cc(CCC(O)=O)ccc1-c1ccc2c(C)nn(-c3ccccn3)c2c1 Show InChI InChI=1S/C23H21N3O3/c1-15-18-10-8-17(14-20(18)26(25-15)22-5-3-4-12-24-22)19-9-6-16(7-11-23(27)28)13-21(19)29-2/h3-6,8-10,12-14H,7,11H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265876
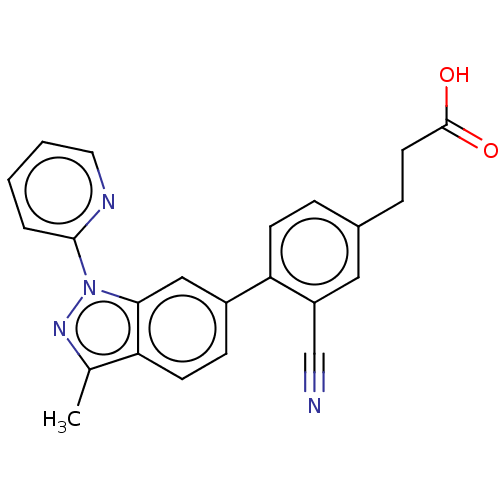
(CHEMBL4077353)Show SMILES Cc1nn(-c2ccccn2)c2cc(ccc12)-c1ccc(CCC(O)=O)cc1C#N Show InChI InChI=1S/C23H18N4O2/c1-15-19-9-7-17(13-21(19)27(26-15)22-4-2-3-11-25-22)20-8-5-16(6-10-23(28)29)12-18(20)14-24/h2-5,7-9,11-13H,6,10H2,1H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265877
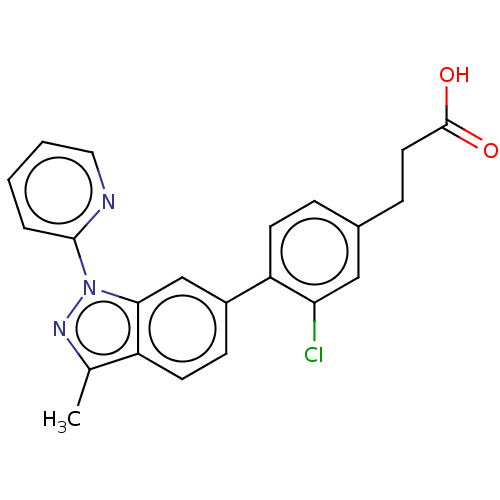
(CHEMBL4101071)Show SMILES Cc1nn(-c2ccccn2)c2cc(ccc12)-c1ccc(CCC(O)=O)cc1Cl Show InChI InChI=1S/C22H18ClN3O2/c1-14-17-9-7-16(13-20(17)26(25-14)21-4-2-3-11-24-21)18-8-5-15(12-19(18)23)6-10-22(27)28/h2-5,7-9,11-13H,6,10H2,1H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 710 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265878
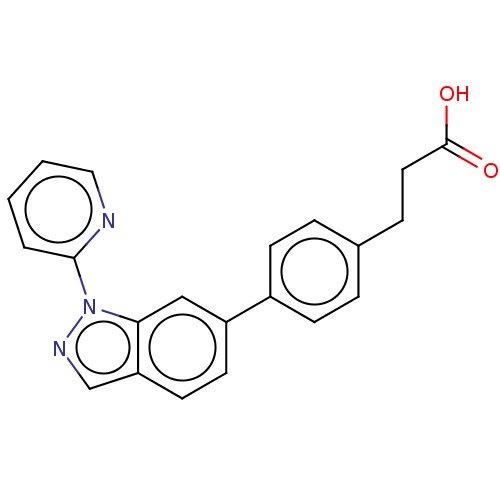
(CHEMBL4060517)Show SMILES OC(=O)CCc1ccc(cc1)-c1ccc2cnn(-c3ccccn3)c2c1 Show InChI InChI=1S/C21H17N3O2/c25-21(26)11-6-15-4-7-16(8-5-15)17-9-10-18-14-23-24(19(18)13-17)20-3-1-2-12-22-20/h1-5,7-10,12-14H,6,11H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 990 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265879
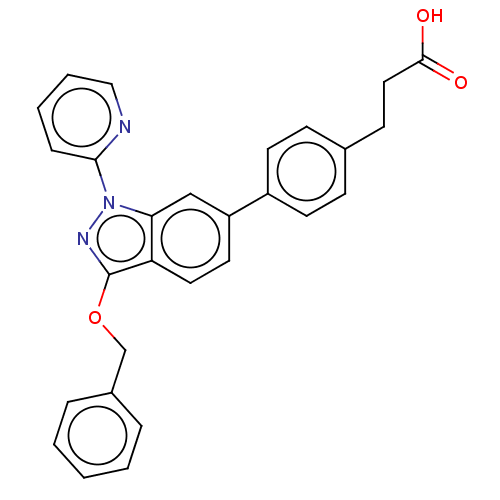
(CHEMBL4065429)Show SMILES OC(=O)CCc1ccc(cc1)-c1ccc2c(OCc3ccccc3)nn(-c3ccccn3)c2c1 Show InChI InChI=1S/C28H23N3O3/c32-27(33)16-11-20-9-12-22(13-10-20)23-14-15-24-25(18-23)31(26-8-4-5-17-29-26)30-28(24)34-19-21-6-2-1-3-7-21/h1-10,12-15,17-18H,11,16,19H2,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.70E+4 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265880
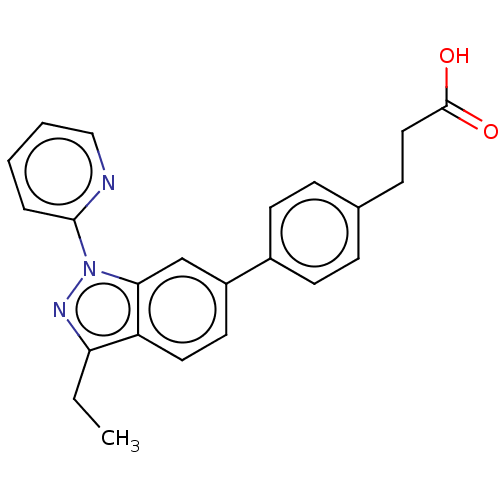
(CHEMBL4076004)Show SMILES CCc1nn(-c2ccccn2)c2cc(ccc12)-c1ccc(CCC(O)=O)cc1 Show InChI InChI=1S/C23H21N3O2/c1-2-20-19-12-11-18(17-9-6-16(7-10-17)8-13-23(27)28)15-21(19)26(25-20)22-5-3-4-14-24-22/h3-7,9-12,14-15H,2,8,13H2,1H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Homo sapiens (Human)) | BDBM50265881
(CHEMBL4065890)Show SMILES OC(=O)CCc1ccc(cc1)-c1ccc2c(nn(-c3ccccn3)c2c1)C#N Show InChI InChI=1S/C22H16N4O2/c23-14-19-18-10-9-17(16-7-4-15(5-8-16)6-11-22(27)28)13-20(18)26(25-19)21-3-1-2-12-24-21/h1-5,7-10,12-13H,6,11H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR120 expressed in CHO-FlpIn cells after 15 mins by DMR assay |
J Med Chem 60: 3187-3197 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00210
BindingDB Entry DOI: 10.7270/Q2PZ5C9Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data