

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50068561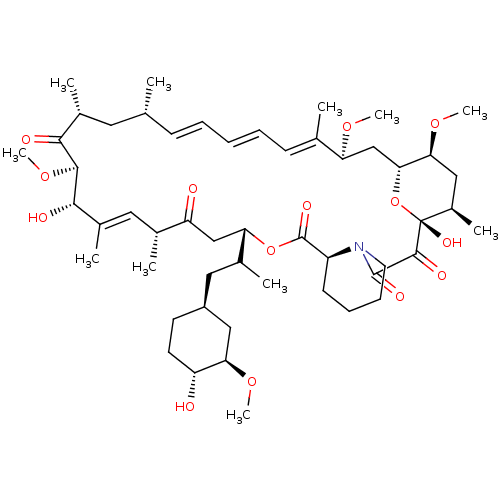 ((16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50174276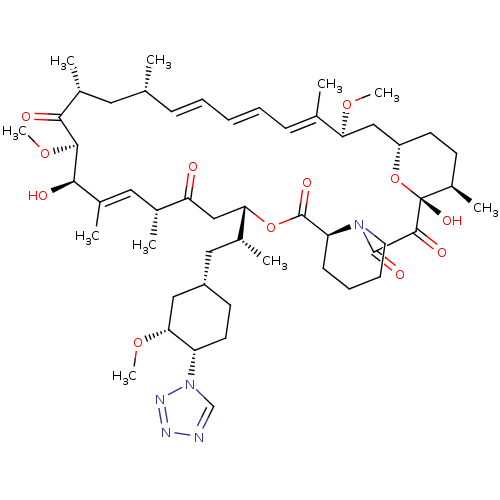 ((1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50174275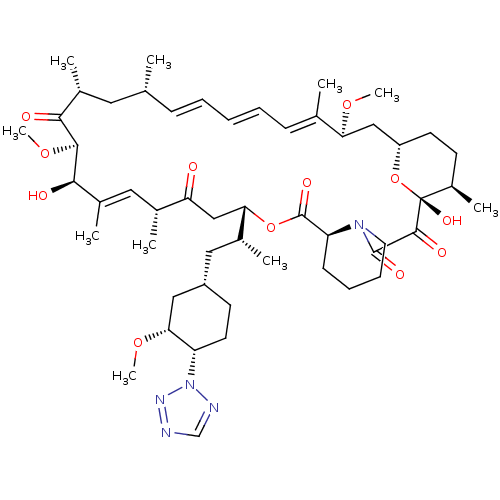 ((1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50174277 ((1R,2R,4S)-4-[(2R)-2-[(1R,9S,15R,16E,18R,19R,21R,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016627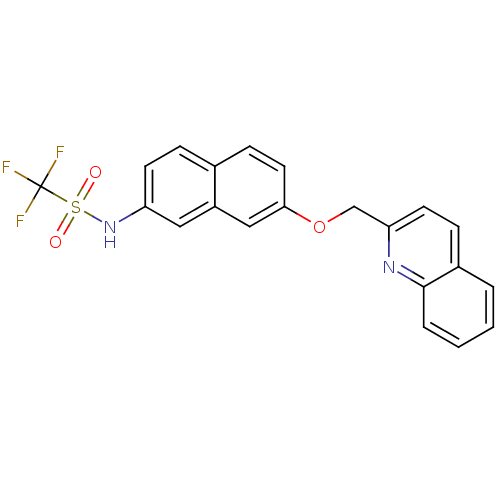 (C,C,C-Trifluoro-N-[7-(quinolin-2-ylmethoxy)-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound to inhibit 5-lipoxygenase in the rat | J Med Chem 32: 1176-83 (1989) BindingDB Entry DOI: 10.7270/Q2833R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50006801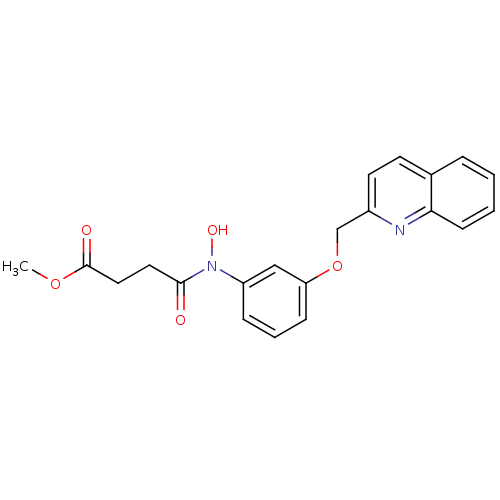 (CHEMBL287012 | N-Hydroxy-N-[3-(quinolin-2-ylmethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration that produces 50% inhibition of A-23,187-stimulated radiolabelled 5-HETE and TXB2 synthesis by PMN 5-lipoxygenase. | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50006806 (C,C,C-Trifluoro-N-[3-(quinolin-2-ylmethoxy)-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration for 50% inhibition of A-23,187-stimulated radiolabelled 5-HETE and TXB2 synthesis by PMN 5-lipoxygenase | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50006806 (C,C,C-Trifluoro-N-[3-(quinolin-2-ylmethoxy)-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration to inhibit Prostaglandin G/H synthase in the rat | J Med Chem 32: 1176-83 (1989) BindingDB Entry DOI: 10.7270/Q2833R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50006820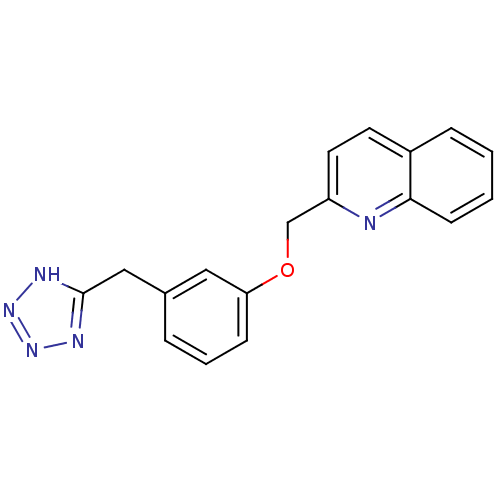 (2-[3-(1H-Tetrazol-5-ylmethyl)-phenoxymethyl]-quino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration that produces 50% inhibition of A-23,187-stimulated radiolabelled 5-HETE and TXB2 synthesis by PMN 5-lipoxygenase | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50006806 (C,C,C-Trifluoro-N-[3-(quinolin-2-ylmethoxy)-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound to inhibit 5-lipoxygenase in the rat | J Med Chem 32: 1176-83 (1989) BindingDB Entry DOI: 10.7270/Q2833R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50006821 (4-Methyl-N-[3-(quinolin-2-ylmethoxy)-benzoyl]-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration that produces 50% inhibition of A-23,187-stimulated radiolabelled 5-HETE and TXB2 synthesis by PMN 5-lipoxygenase. | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50006806 (C,C,C-Trifluoro-N-[3-(quinolin-2-ylmethoxy)-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration for 50% inhibition of A-23187-stimulated radiolabeled 5-HETE and TXB2 synthesis by Prostaglandin G/H synthase | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50016627 (C,C,C-Trifluoro-N-[7-(quinolin-2-ylmethoxy)-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration to inhibit Prostaglandin G/H synthase in the rat | J Med Chem 32: 1176-83 (1989) BindingDB Entry DOI: 10.7270/Q2833R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50006821 (4-Methyl-N-[3-(quinolin-2-ylmethoxy)-benzoyl]-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration that produces 50% inhibition of A-23187-stimulated radiolabeled 5-HETE and TXB2 synthesis by Prostaglandin G/H synthase | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50034545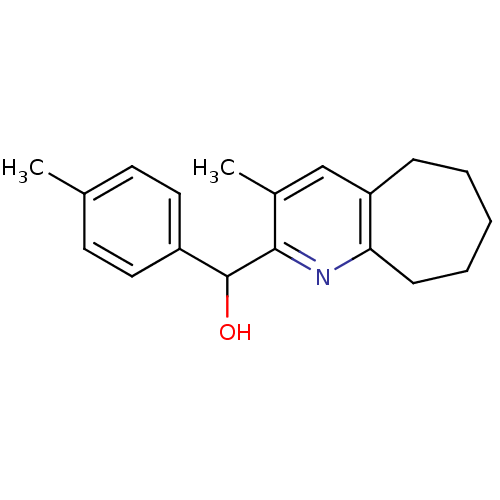 ((3-Methyl-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research (U.K.) Ltd. Curated by ChEMBL | Assay Description 5-lipoxygenase inhibitory activity against rat polymorphonuclear leucocytes from female wistar rat, by using LTB4 . | J Med Chem 38: 1473-81 (1995) BindingDB Entry DOI: 10.7270/Q2VH5MV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50006813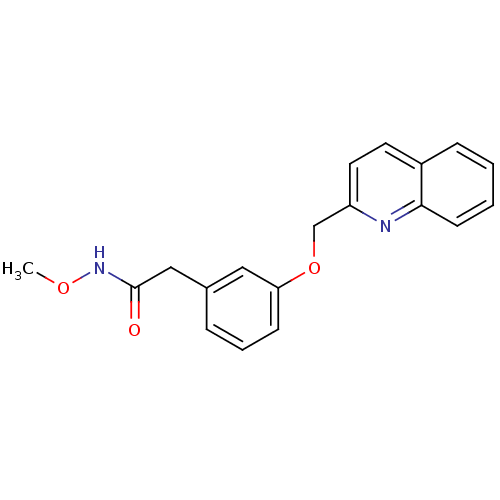 (CHEMBL79453 | N-Methoxy-2-[3-(quinolin-2-ylmethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration for 50% inhibition of A-23,187-stimulated radiolabelled 5-HETE and TXB2 synthesis by PMN 5-lipoxygenase | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50006816 ((LY-171883)1-{2-Hydroxy-3-propyl-4-[4-(1H-tetrazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration for 50% inhibition of A-23,187-stimulated radiolabelled 5-HETE and TXB2 synthesis by PMN 5-lipoxygenase | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50034545 ((3-Methyl-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research (U.K.) Ltd. Curated by ChEMBL | Assay Description 5-lipoxygenase inhibitory activity against rat polymorphonuclear leucocytes from female wistar rat, by using 5-HETE. | J Med Chem 38: 1473-81 (1995) BindingDB Entry DOI: 10.7270/Q2VH5MV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50006801 (CHEMBL287012 | N-Hydroxy-N-[3-(quinolin-2-ylmethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration that produces 50% inhibition of A-23187-stimulated radiolabeled 5-HETE and TXB2 synthesis by PMN (Prostaglandin G/H synthase). | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50006816 ((LY-171883)1-{2-Hydroxy-3-propyl-4-[4-(1H-tetrazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration for 50% inhibition of A-23187-stimulated radiolabeled 5-HETE and TXB2 synthesis by Prostaglandin G/H synthase | J Med Chem 33: 240-5 (1990) BindingDB Entry DOI: 10.7270/Q24Q7T0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||