 Found 194 hits with Last Name = 'cass' and Initial = 'r'
Found 194 hits with Last Name = 'cass' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590202
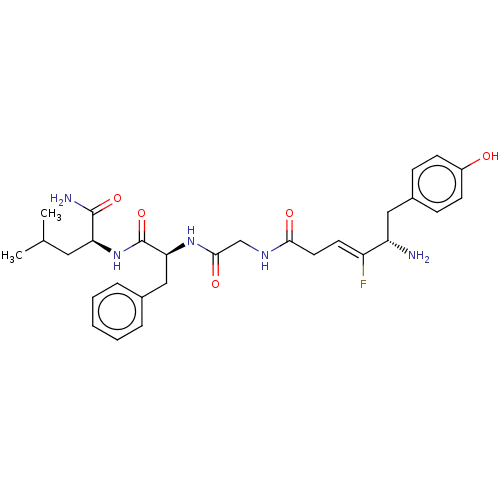
(CHEMBL5192115)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590202

(CHEMBL5192115)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590201
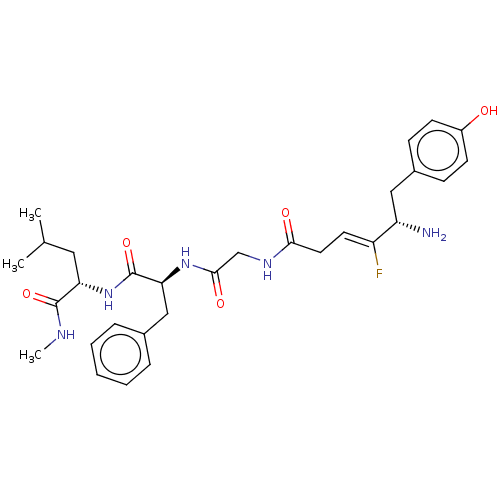
(CHEMBL5173456)Show SMILES CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590201

(CHEMBL5173456)Show SMILES CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590200
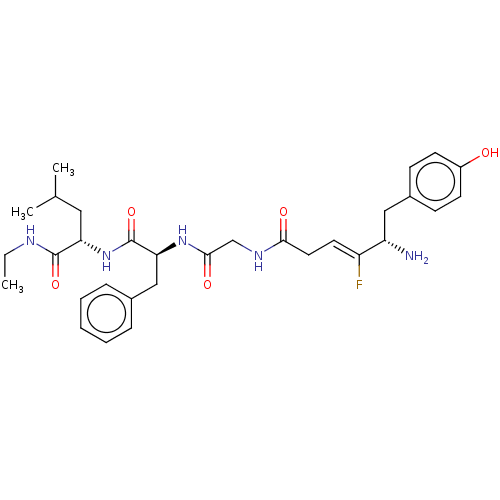
(CHEMBL5198168)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590200

(CHEMBL5198168)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50517887
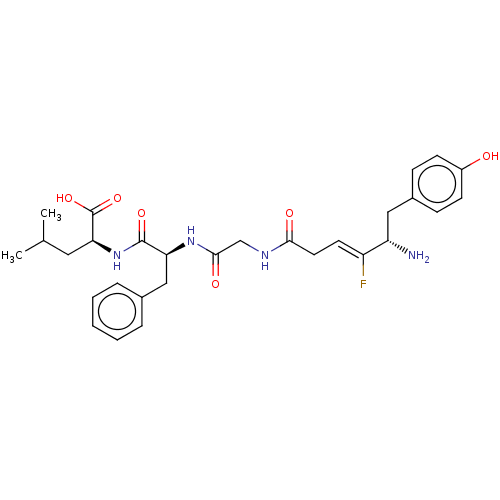
(CHEMBL4531563)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C29H37FN4O6/c1-18(2)14-25(29(39)40)34-28(38)24(16-19-6-4-3-5-7-19)33-27(37)17-32-26(36)13-12-22(30)23(31)15-20-8-10-21(35)11-9-20/h3-12,18,23-25,35H,13-17,31H2,1-2H3,(H,32,36)(H,33,37)(H,34,38)(H,39,40)/b22-12-/t23-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590199
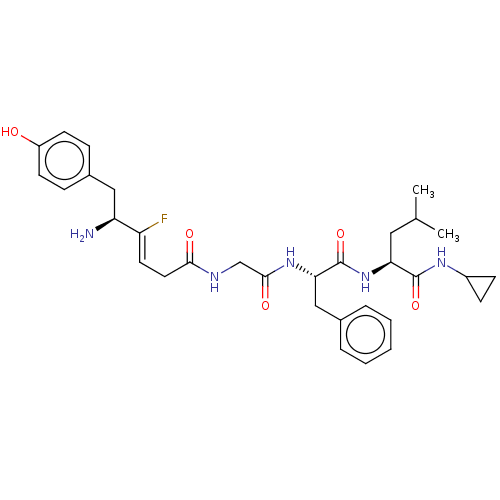
(CHEMBL5172639)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(=O)NC1CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590199

(CHEMBL5172639)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(=O)NC1CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50517887

(CHEMBL4531563)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C29H37FN4O6/c1-18(2)14-25(29(39)40)34-28(38)24(16-19-6-4-3-5-7-19)33-27(37)17-32-26(36)13-12-22(30)23(31)15-20-8-10-21(35)11-9-20/h3-12,18,23-25,35H,13-17,31H2,1-2H3,(H,32,36)(H,33,37)(H,34,38)(H,39,40)/b22-12-/t23-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50517887

(CHEMBL4531563)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C29H37FN4O6/c1-18(2)14-25(29(39)40)34-28(38)24(16-19-6-4-3-5-7-19)33-27(37)17-32-26(36)13-12-22(30)23(31)15-20-8-10-21(35)11-9-20/h3-12,18,23-25,35H,13-17,31H2,1-2H3,(H,32,36)(H,33,37)(H,34,38)(H,39,40)/b22-12-/t23-,24-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50517887

(CHEMBL4531563)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C29H37FN4O6/c1-18(2)14-25(29(39)40)34-28(38)24(16-19-6-4-3-5-7-19)33-27(37)17-32-26(36)13-12-22(30)23(31)15-20-8-10-21(35)11-9-20/h3-12,18,23-25,35H,13-17,31H2,1-2H3,(H,32,36)(H,33,37)(H,34,38)(H,39,40)/b22-12-/t23-,24-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590200

(CHEMBL5198168)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590200

(CHEMBL5198168)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590201

(CHEMBL5173456)Show SMILES CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590201

(CHEMBL5173456)Show SMILES CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590198
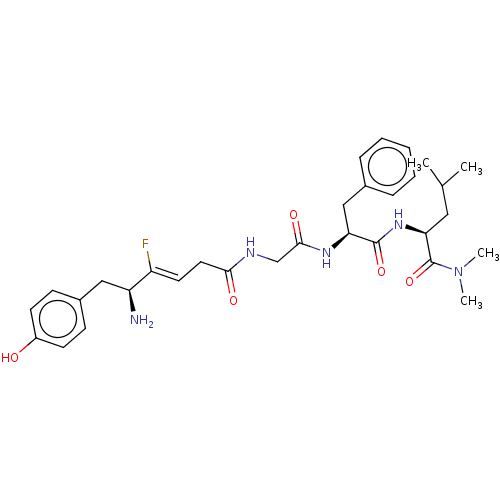
(CHEMBL5203813)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(=O)N(C)C |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590198

(CHEMBL5203813)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(=O)N(C)C |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590202

(CHEMBL5192115)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590202

(CHEMBL5192115)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590199

(CHEMBL5172639)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(=O)NC1CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590199

(CHEMBL5172639)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(=O)NC1CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590198

(CHEMBL5203813)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(=O)N(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590198

(CHEMBL5203813)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)C(=O)N(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590203
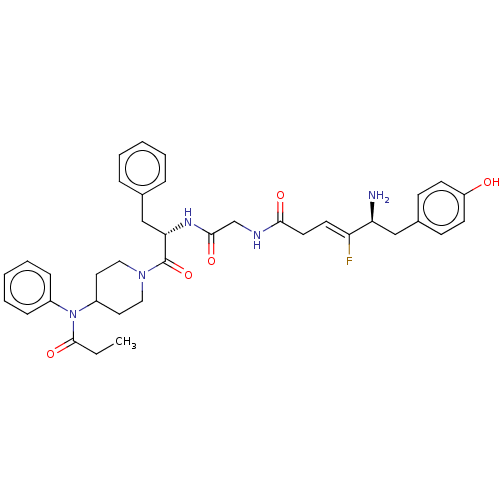
(CHEMBL5177001)Show SMILES CCC(=O)N(C1CCN(CC1)C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 368 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590203

(CHEMBL5177001)Show SMILES CCC(=O)N(C1CCN(CC1)C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50590203

(CHEMBL5177001)Show SMILES CCC(=O)N(C1CCN(CC1)C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50590203

(CHEMBL5177001)Show SMILES CCC(=O)N(C1CCN(CC1)C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)C\C=C(/F)[C@@H](N)Cc1ccc(O)cc1)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50471296

(CHEMBL87935)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](Cc2nc(no2)-c2ccccc2)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C21H28N2O6/c1-11(12(2)24)20-16(28-20)8-14-10-27-15(19(26)18(14)25)9-17-22-21(23-29-17)13-6-4-3-5-7-13/h3-7,11-12,14-16,18-20,24-26H,8-10H2,1-2H3/t11-,12-,14-,15-,16-,18+,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 |
J Med Chem 40: 2563-70 (1997)
Article DOI: 10.1021/jm960738k
BindingDB Entry DOI: 10.7270/Q2GH9MPZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50290686

(9-((E)-4-((2S,3R,4R,5S)-3,4-dihydroxy-5-(((2S,3S)-...)Show SMILES C[C@H](O)[C@H](C)[C@@H]1O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\C(=O)OCCCCCCCCC(O)=O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C26H44O9/c1-16(13-23(30)33-11-9-7-5-4-6-8-10-22(28)29)12-20-25(32)24(31)19(15-34-20)14-21-26(35-21)17(2)18(3)27/h13,17-21,24-27,31-32H,4-12,14-15H2,1-3H3,(H,28,29)/b16-13+/t17-,18-,19-,20-,21-,24+,25-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 |
J Med Chem 40: 2563-70 (1997)
Article DOI: 10.1021/jm960738k
BindingDB Entry DOI: 10.7270/Q2GH9MPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(MOUSE) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50471292

(CHEMBL315230)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\C(=O)OCC)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C19H32O7/c1-5-24-16(21)7-10(2)6-14-18(23)17(22)13(9-25-14)8-15-19(26-15)11(3)12(4)20/h7,11-15,17-20,22-23H,5-6,8-9H2,1-4H3/b10-7+/t11-,12-,13-,14-,15-,17+,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 |
J Med Chem 40: 2563-70 (1997)
Article DOI: 10.1021/jm960738k
BindingDB Entry DOI: 10.7270/Q2GH9MPZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50471294

(CHEMBL276855)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](Cc2cnc(o2)-c2ccccc2)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C22H29NO6/c1-12(13(2)24)21-18(29-21)8-15-11-27-17(20(26)19(15)25)9-16-10-23-22(28-16)14-6-4-3-5-7-14/h3-7,10,12-13,15,17-21,24-26H,8-9,11H2,1-2H3/t12-,13-,15-,17-,18-,19+,20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 |
J Med Chem 40: 2563-70 (1997)
Article DOI: 10.1021/jm960738k
BindingDB Entry DOI: 10.7270/Q2GH9MPZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50471297

(CHEMBL424608)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](Cc2nnc(o2)-c2ccccc2)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C21H28N2O6/c1-11(12(2)24)20-16(28-20)8-14-10-27-15(19(26)18(14)25)9-17-22-23-21(29-17)13-6-4-3-5-7-13/h3-7,11-12,14-16,18-20,24-26H,8-10H2,1-2H3/t11-,12-,14-,15-,16-,18+,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 |
J Med Chem 40: 2563-70 (1997)
Article DOI: 10.1021/jm960738k
BindingDB Entry DOI: 10.7270/Q2GH9MPZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50471293

(CHEMBL83449)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](Cn2nnc(n2)-c2ccccc2)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C20H28N4O5/c1-11(12(2)25)19-15(29-19)8-14-10-28-16(18(27)17(14)26)9-24-22-20(21-23-24)13-6-4-3-5-7-13/h3-7,11-12,14-19,25-27H,8-10H2,1-2H3/t11-,12-,14-,15-,16-,17+,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 |
J Med Chem 40: 2563-70 (1997)
Article DOI: 10.1021/jm960738k
BindingDB Entry DOI: 10.7270/Q2GH9MPZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50470911

(CHEMBL122825)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\c2ncc(o2)-c2csc(c2)[N+]([O-])=O)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C23H30N2O8S/c1-11(5-19-24-8-18(32-19)15-7-20(25(29)30)34-10-15)4-16-22(28)21(27)14(9-31-16)6-17-23(33-17)12(2)13(3)26/h5,7-8,10,12-14,16-17,21-23,26-28H,4,6,9H2,1-3H3/b11-5+/t12-,13-,14-,16-,17-,21+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.45 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus |
J Med Chem 39: 3596-600 (1996)
Article DOI: 10.1021/jm950882q
BindingDB Entry DOI: 10.7270/Q2TX3J3G |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50471295

(CHEMBL312853)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](Cc2cc(no2)-c2ccccc2)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C22H29NO6/c1-12(13(2)24)22-19(28-22)8-15-11-27-18(21(26)20(15)25)10-16-9-17(23-29-16)14-6-4-3-5-7-14/h3-7,9,12-13,15,18-22,24-26H,8,10-11H2,1-2H3/t12-,13-,15-,18-,19-,20+,21-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 |
J Med Chem 40: 2563-70 (1997)
Article DOI: 10.1021/jm960738k
BindingDB Entry DOI: 10.7270/Q2GH9MPZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50471291

(CHEMBL83394)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](Cc2csc(n2)-c2ccccc2)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C22H29NO5S/c1-12(13(2)24)21-18(28-21)8-15-10-27-17(20(26)19(15)25)9-16-11-29-22(23-16)14-6-4-3-5-7-14/h3-7,11-13,15,17-21,24-26H,8-10H2,1-2H3/t12-,13-,15-,17-,18-,19+,20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition concentration against isoleucyl tRNA synthetase from Staphylococcus aureus NCTC 6571 |
J Med Chem 40: 2563-70 (1997)
Article DOI: 10.1021/jm960738k
BindingDB Entry DOI: 10.7270/Q2GH9MPZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50470914
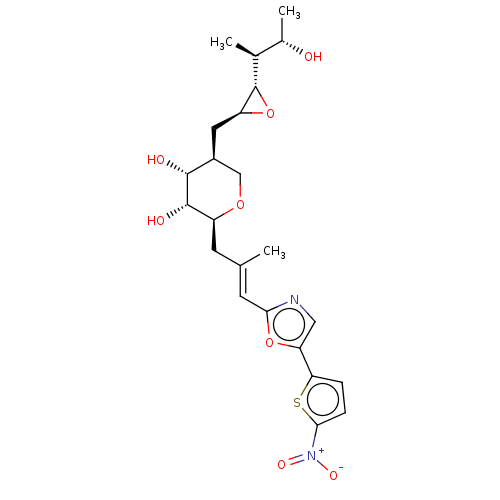
(CHEMBL120343)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\c2ncc(o2)-c2ccc(s2)[N+]([O-])=O)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C23H30N2O8S/c1-11(7-19-24-9-17(32-19)18-4-5-20(34-18)25(29)30)6-15-22(28)21(27)14(10-31-15)8-16-23(33-16)12(2)13(3)26/h4-5,7,9,12-16,21-23,26-28H,6,8,10H2,1-3H3/b11-7+/t12-,13-,14-,15-,16-,21+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.67 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus NCTC 3571 |
J Med Chem 39: 3596-600 (1996)
Article DOI: 10.1021/jm950882q
BindingDB Entry DOI: 10.7270/Q2TX3J3G |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50470913
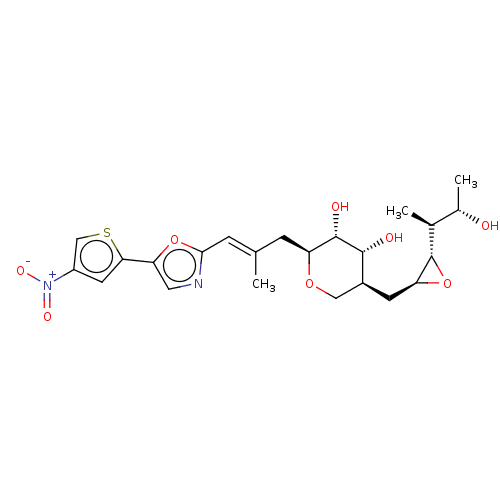
(CHEMBL120798)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\c2ncc(o2)-c2cc(cs2)[N+]([O-])=O)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C23H30N2O8S/c1-11(5-20-24-8-18(32-20)19-7-15(10-34-19)25(29)30)4-16-22(28)21(27)14(9-31-16)6-17-23(33-17)12(2)13(3)26/h5,7-8,10,12-14,16-17,21-23,26-28H,4,6,9H2,1-3H3/b11-5+/t12-,13-,14-,16-,17-,21+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.87 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus |
J Med Chem 39: 3596-600 (1996)
Article DOI: 10.1021/jm950882q
BindingDB Entry DOI: 10.7270/Q2TX3J3G |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50470912

(CHEMBL120437)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\c2ncc(o2)-c2ccc(cc2)[N+]([O-])=O)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C25H32N2O8/c1-13(9-22-26-11-21(34-22)16-4-6-18(7-5-16)27(31)32)8-19-24(30)23(29)17(12-33-19)10-20-25(35-20)14(2)15(3)28/h4-7,9,11,14-15,17,19-20,23-25,28-30H,8,10,12H2,1-3H3/b13-9+/t14-,15-,17-,19-,20-,23+,24-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.98 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus |
J Med Chem 39: 3596-600 (1996)
Article DOI: 10.1021/jm950882q
BindingDB Entry DOI: 10.7270/Q2TX3J3G |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00025j
BindingDB Entry DOI: 10.7270/Q28P64HN |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50321325

(CHEMBL4173485)Show InChI InChI=1S/C7H6N2OS/c8-7-6(3-9-10-7)5-1-2-11-4-5/h1-4H,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO2 assessed as decrease in conversion of L-tryptophan to N-formylkynurenine preincubated for 5 mins followed by 0.2... |
ACS Med Chem Lett 9: 417-421 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00427
BindingDB Entry DOI: 10.7270/Q2M0481G |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50321332

(CHEMBL4165554)Show InChI InChI=1S/C7H6N2OS/c8-7-5(4-9-10-7)6-2-1-3-11-6/h1-4H,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO2 assessed as decrease in conversion of L-tryptophan to N-formylkynurenine preincubated for 5 mins followed by 0.2... |
ACS Med Chem Lett 9: 417-421 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00427
BindingDB Entry DOI: 10.7270/Q2M0481G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data