

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Lactobacillus casei) | BDBM50022238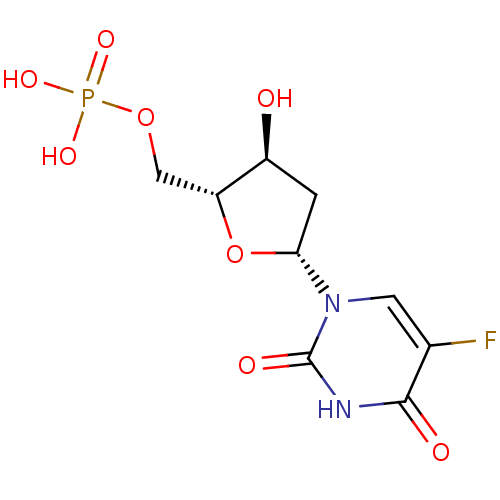 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010241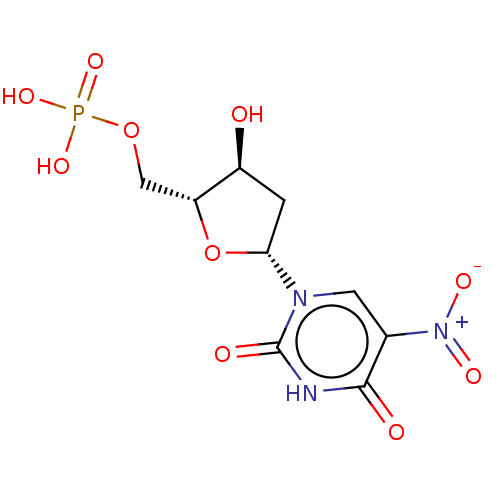 (CHEMBL1234672) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010236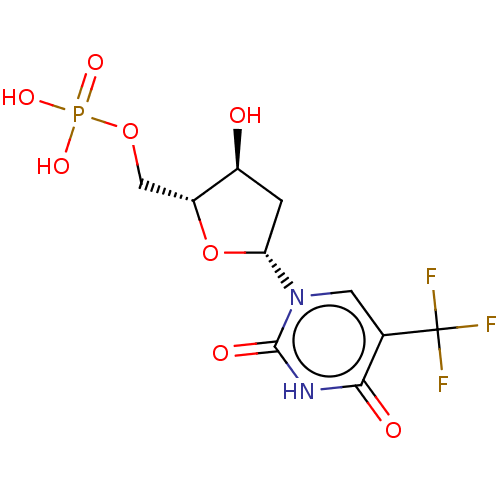 (CHEMBL3144200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50000038 (CHEMBL3228321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010239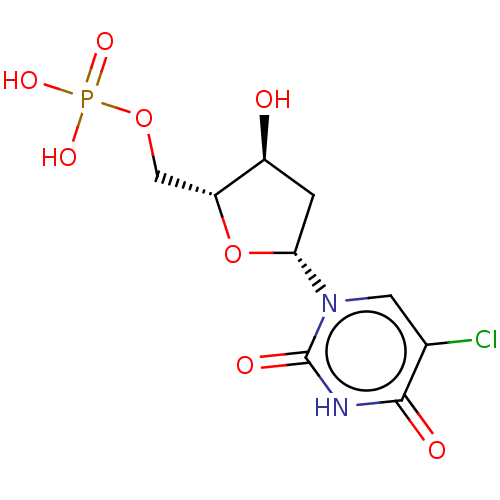 (CHEMBL1236538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50404974 (5-NITRO-DUMP | CHEMBL2051758) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inactivation of thymidylate synthetase measured asKi at 6.8 pH 30 degrees centigrade temp | J Med Chem 26: 1028-36 (1983) BindingDB Entry DOI: 10.7270/Q2VD6XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50027919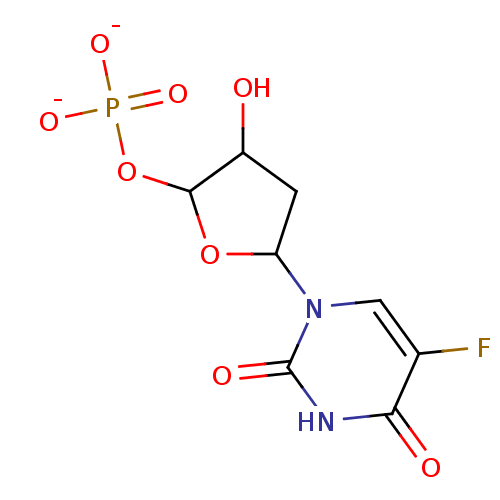 (5-fluoro-deoxyuridinemonophosphate | CHEMBL416879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inactivation of thymidylate synthetase measured asKi at 6.8 pH 30 degrees centigrade temp | J Med Chem 26: 1028-36 (1983) BindingDB Entry DOI: 10.7270/Q2VD6XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010240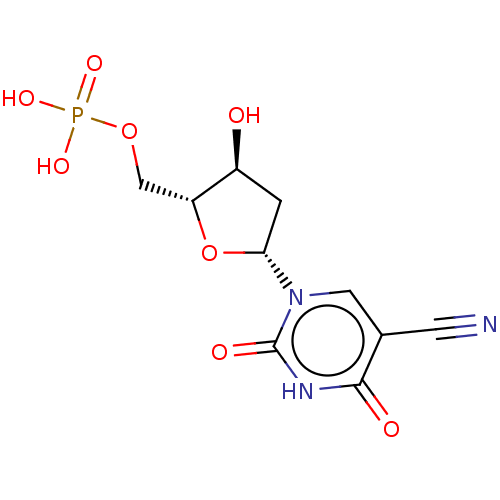 (CHEMBL3246102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010238 (CHEMBL1160593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010242 (CHEMBL1160594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50027920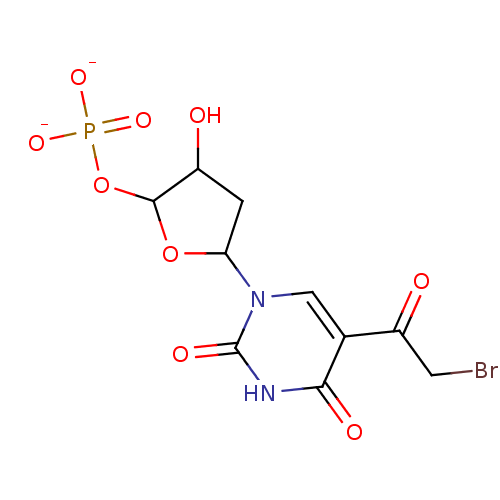 (5-quinonyl-deoxyuridinemonophosphate | CHEMBL8016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inactivation of thymidylate synthetase measured asKi at 6.8 pH 30 degrees centigrade temp | J Med Chem 26: 1028-36 (1983) BindingDB Entry DOI: 10.7270/Q2VD6XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50000019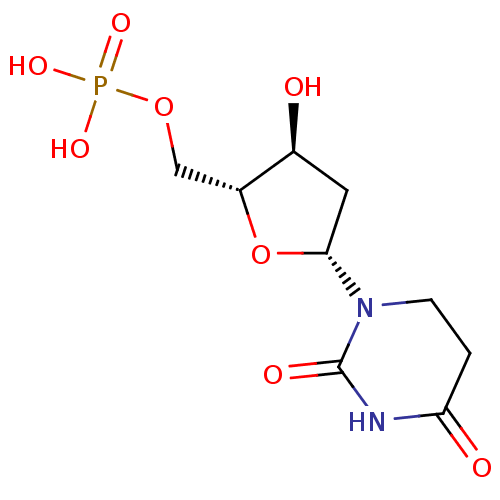 (CHEMBL3144338) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Lactobacillus casei thymidylate synthetase using dTMP as substrate assessed as release of water after 15 mins by double rec... | J Med Chem 22: 319-21 (1979) BindingDB Entry DOI: 10.7270/Q26111TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50404975 (CHEMBL2051976) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inactivation of thymidylate synthetase measured as Ki at 6.8 pH 30 degrees centigrade temp | J Med Chem 26: 1028-36 (1983) BindingDB Entry DOI: 10.7270/Q2VD6XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50000019 (CHEMBL3144338) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Lactobacillus casei thymidylate synthetase using 2'-deoxy[5-3H]uridine-5'-phosphate as substrate assessed as release of wat... | J Med Chem 22: 319-21 (1979) BindingDB Entry DOI: 10.7270/Q26111TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50000034 (CHEMBL3228124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Lactobacillus casei thymidylate synthetase using 2'-deoxy[5-3H]uridine-5'-phosphate as substrate assessed as release of wat... | J Med Chem 22: 319-21 (1979) BindingDB Entry DOI: 10.7270/Q26111TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-5/beta-1 (Homo sapiens (Human)) | BDBM50033030 ((R)-3-{2-[(R)-2-Oxo-3-(2-piperidin-4-yl-ethyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human umbilical vein endothelial cell adhesion to fibronectin | J Med Chem 38: 3332-41 (1995) BindingDB Entry DOI: 10.7270/Q23N24K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50033030 ((R)-3-{2-[(R)-2-Oxo-3-(2-piperidin-4-yl-ethyl)-pip...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human umbilical vein endothelial cell adhesion to fibrinogen | J Med Chem 38: 3332-41 (1995) BindingDB Entry DOI: 10.7270/Q23N24K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50033030 ((R)-3-{2-[(R)-2-Oxo-3-(2-piperidin-4-yl-ethyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human umbilical vein endothelial cell adhesion to vitronectin | J Med Chem 38: 3332-41 (1995) BindingDB Entry DOI: 10.7270/Q23N24K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50004058 ((2S)-2-(butylsulfonylamino)-3-[4-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of protein kinase C | Bioorg Med Chem Lett 9: 2279-82 (1999) BindingDB Entry DOI: 10.7270/Q2MW2K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039593 ((S)-3-[4-(4-Piperidin-4-yl-butoxy)-phenyl]-2-(thio...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50004071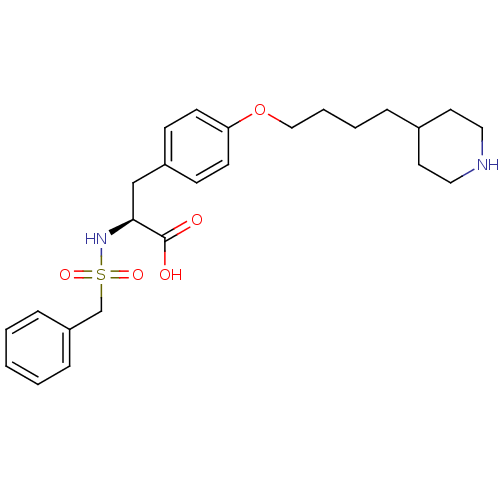 ((S)-2-Phenylmethanesulfonylamino-3-[4-(4-piperidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039590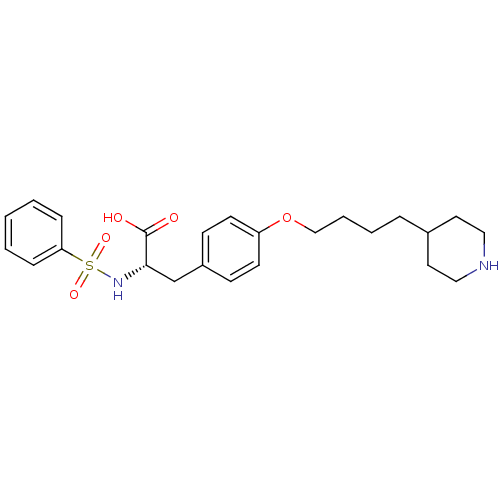 ((S)-2-Benzenesulfonylamino-3-[4-(4-piperidin-4-yl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039592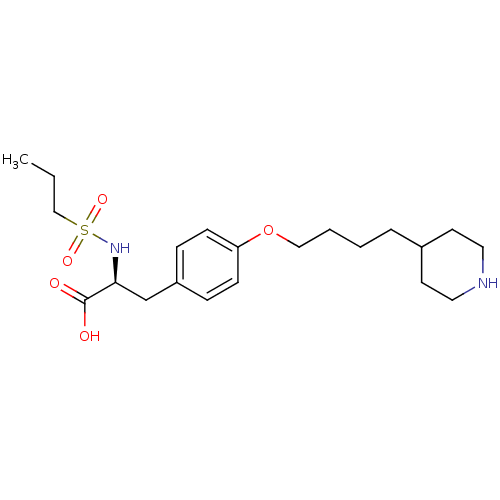 ((S)-3-[4-(4-Piperidin-4-yl-butoxy)-phenyl]-2-(prop...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039580 ((S)-2-(2-Phenyl-ethanesulfonylamino)-3-[4-(4-piper...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50004061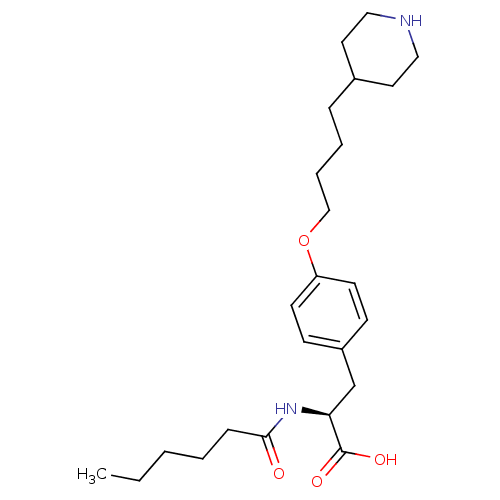 ((S)-2-Hexanoylamino-3-[4-(4-piperidin-4-yl-butoxy)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039576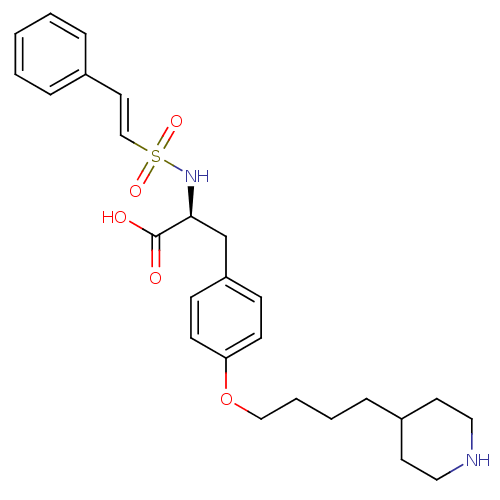 ((S)-2-((E)-2-Phenyl-ethenesulfonylamino)-3-[4-(4-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039572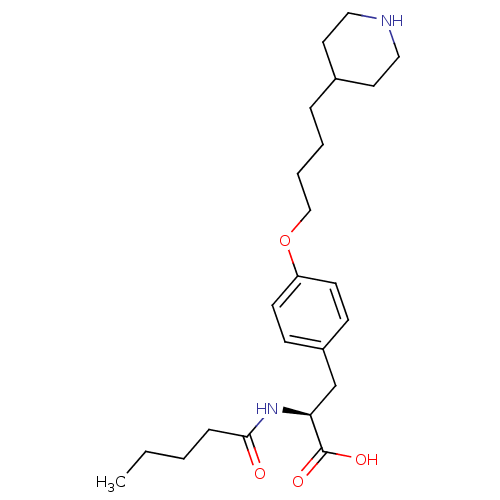 ((S)-2-Pentanoylamino-3-[4-(4-piperidin-4-yl-butoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039591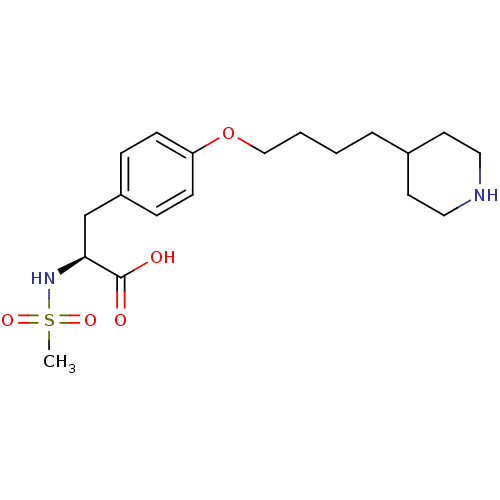 ((S)-2-Methanesulfonylamino-3-[4-(4-piperidin-4-yl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039595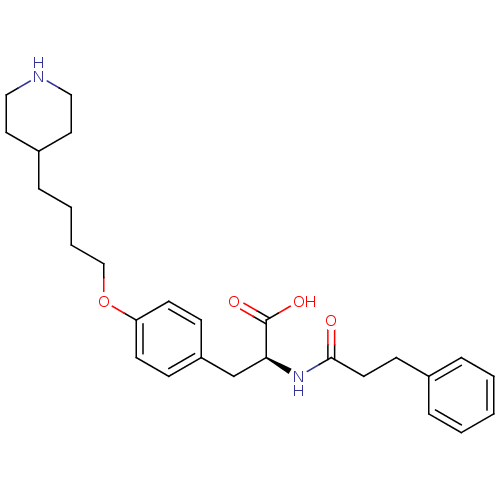 ((S)-2-(3-Phenyl-propionylamino)-3-[4-(4-piperidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039570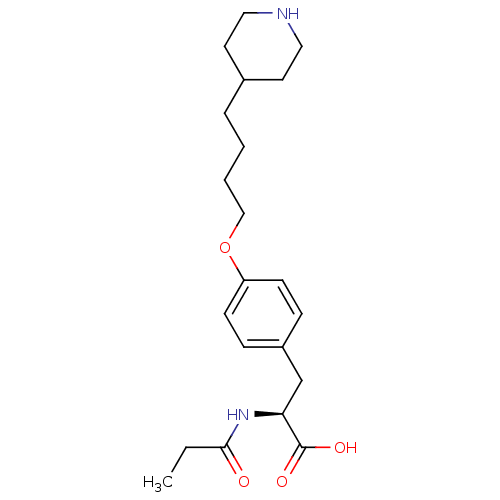 ((S)-3-[4-(4-Piperidin-4-yl-butoxy)-phenyl]-2-propi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039569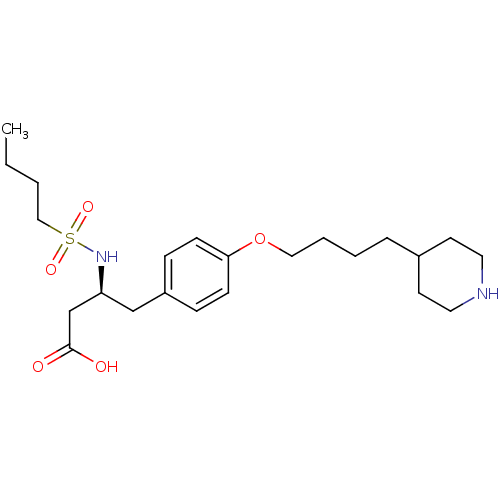 ((S)-3-(Butane-1-sulfonylamino)-4-[4-(4-piperidin-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039586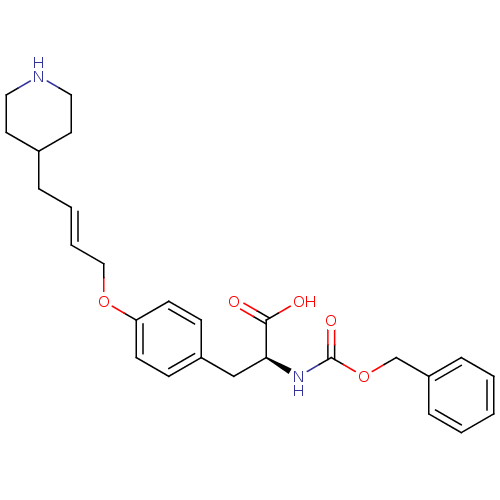 ((S)-2-Benzyloxycarbonylamino-3-[4-((E)-4-piperidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50004060 ((S)-2-Benzyloxycarbonylamino-3-[4-(4-piperidin-4-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50217039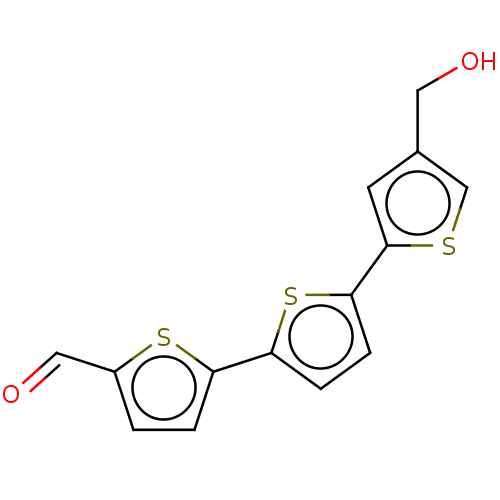 (CHEMBL420938) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of protein kinase C | Bioorg Med Chem Lett 9: 2279-82 (1999) BindingDB Entry DOI: 10.7270/Q2MW2K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50431178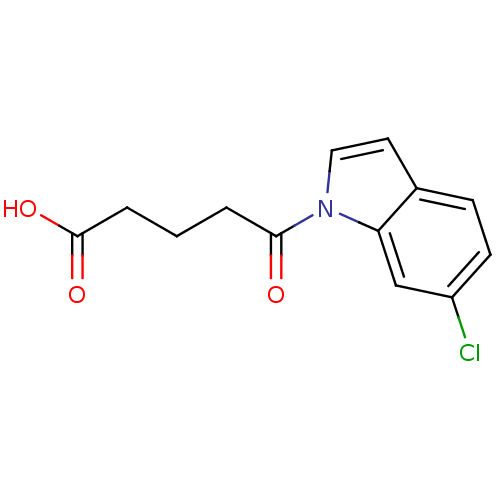 (CHEMBL2332562) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as 5-oxo-ETE-induced Ca2+ mobilization incubated for 2 mins prior to 5-oxo-ETE addi... | J Med Chem 56: 3725-32 (2013) Article DOI: 10.1021/jm400480j BindingDB Entry DOI: 10.7270/Q2RB75XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039588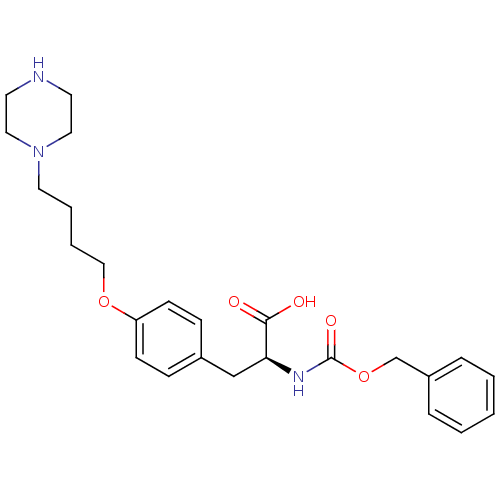 ((S)-2-Benzyloxycarbonylamino-3-[4-(4-piperazin-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039597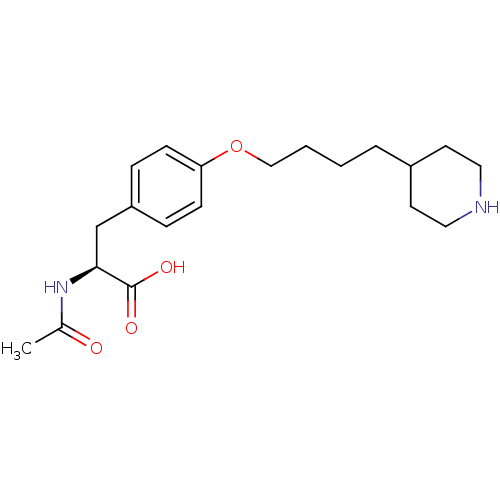 ((S)-2-Acetylamino-3-[4-(4-piperidin-4-yl-butoxy)-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039568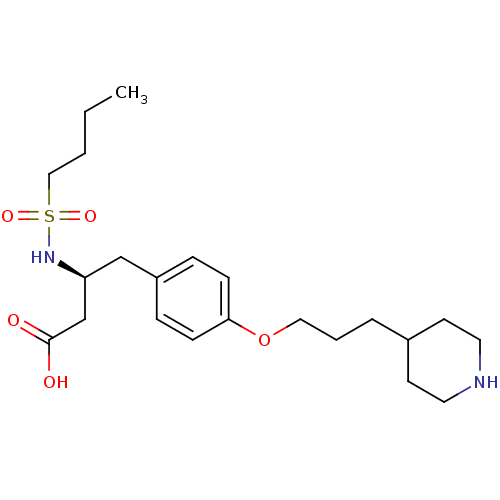 ((S)-3-(Butane-1-sulfonylamino)-4-[4-(3-piperidin-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039601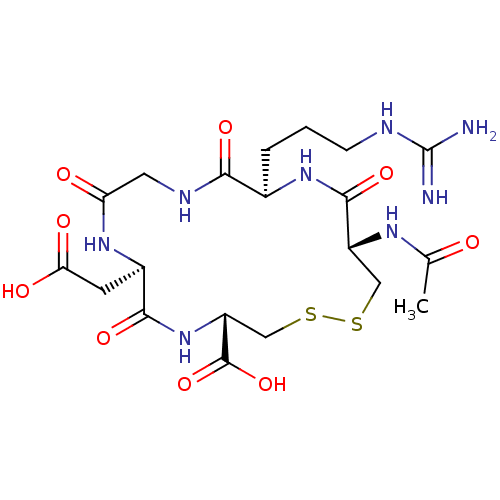 ((4S,7R,13R,16S)-16-Acetylamino-7-carboxymethyl-13-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50217036 (CHEMBL74983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of protein kinase C | Bioorg Med Chem Lett 9: 2279-82 (1999) BindingDB Entry DOI: 10.7270/Q2MW2K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50004065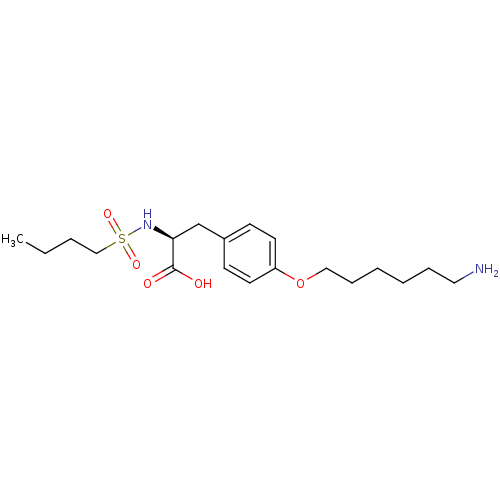 ((S)-3-[4-(6-Amino-hexyloxy)-phenyl]-2-(butane-1-su...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039584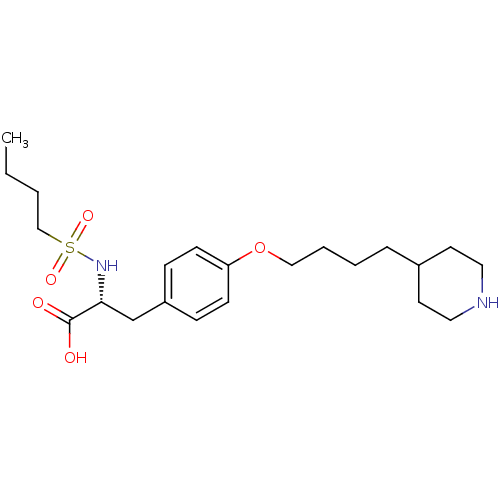 ((R)-2-(Butane-1-sulfonylamino)-3-[4-(4-piperidin-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039577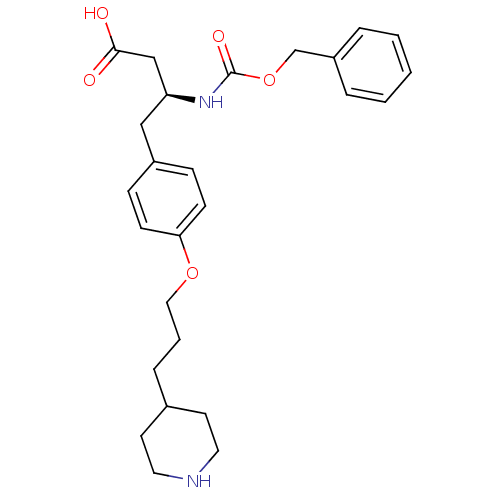 ((S)-3-Benzyloxycarbonylamino-4-[4-(3-piperidin-4-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039571 ((S)-2-Benzyloxycarbonylamino-3-[4-(5-piperazin-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50431179 (CHEMBL2332561) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as 5-oxo-ETE-induced Ca2+ mobilization incubated for 2 mins prior to 5-oxo-ETE addi... | J Med Chem 56: 3725-32 (2013) Article DOI: 10.1021/jm400480j BindingDB Entry DOI: 10.7270/Q2RB75XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039581 ((S)-2-Hexanoylamino-3-[4-(5-piperidin-4-yl-pentyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50431184 (CHEMBL2332567) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as 5-oxo-ETE-induced Ca2+ mobilization incubated for 2 mins prior to 5-oxo-ETE addi... | J Med Chem 56: 3725-32 (2013) Article DOI: 10.1021/jm400480j BindingDB Entry DOI: 10.7270/Q2RB75XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50039573 (2-[1-[1-amino-4-amino(imino)methylamino-(1S)-butyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50004066 ((S)-3-[4-(6-Amino-hexyloxy)-phenyl]-2-phenylmethan...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of platelet aggregation as the concentration necessary to inhibit the change in light . | J Med Chem 37: 2537-51 (1994) BindingDB Entry DOI: 10.7270/Q2N878VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 140 total ) | Next | Last >> |