

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BAK from His-tagged BCL-XL (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50022583 (CHEMBL3298854) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BID from His-tagged MCL-1 (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50022622 (CHEMBL3299033) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BID from His-tagged MCL-1 (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50022568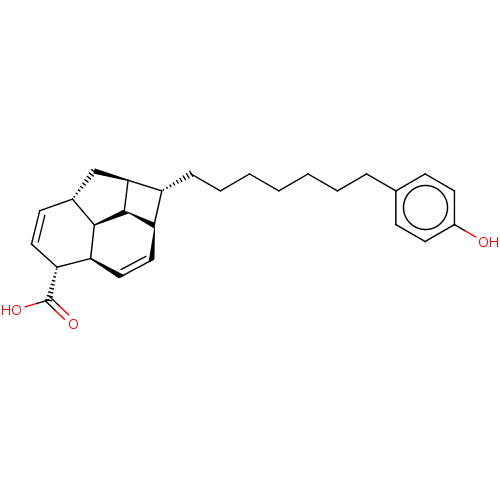 (CHEMBL3298853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BAK from His-tagged BCL-XL (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50022568 (CHEMBL3298853) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BID from His-tagged MCL-1 (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50022567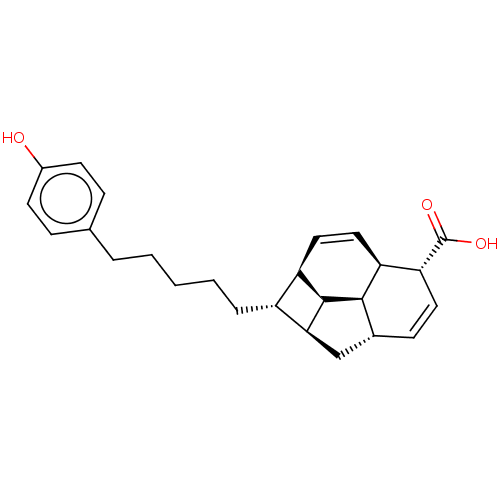 (CHEMBL3298852) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BID from His-tagged MCL-1 (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50022567 (CHEMBL3298852) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BAK from His-tagged BCL-XL (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50022622 (CHEMBL3299033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BAK from His-tagged BCL-XL (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BID from His-tagged MCL-1 (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50022583 (CHEMBL3298854) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BAK from His-tagged BCL-XL (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50022655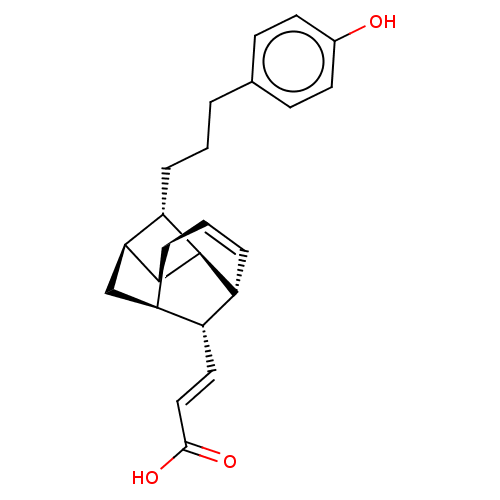 (CHEMBL3299034) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BAK from His-tagged BCL-XL (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50022566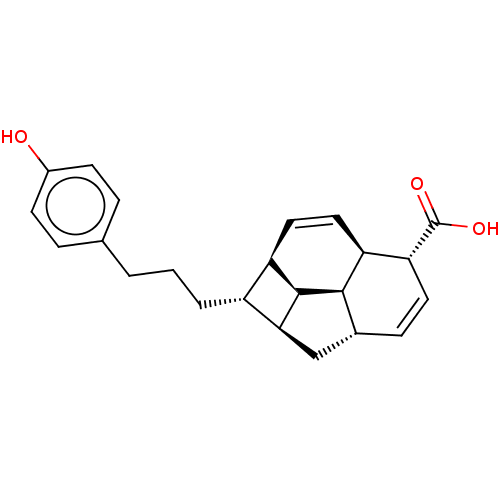 (CHEMBL3298851) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles (ICSN) Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled BAK from His-tagged BCL-XL (unknown origin) after 1 hr by fluorescence polarization assay | J Nat Prod 77: 1430-7 (2014) Article DOI: 10.1021/np500170v BindingDB Entry DOI: 10.7270/Q2R212XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus C2888 AChE using acetylthiocholine iodide as substrate by Ellman's spectroscopic method | J Nat Prod 75: 2012-2015 (2012) Article DOI: 10.1021/np300660y BindingDB Entry DOI: 10.7270/Q2959JNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50394654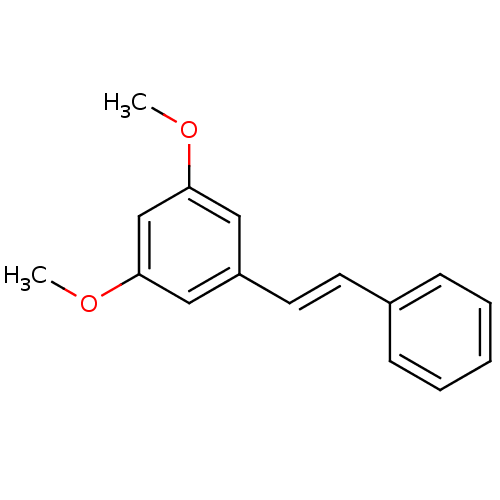 (CHEMBL188181) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus C2888 AChE using acetylthiocholine iodide as substrate by Ellman's spectroscopic method | J Nat Prod 75: 2012-2015 (2012) Article DOI: 10.1021/np300660y BindingDB Entry DOI: 10.7270/Q2959JNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50394652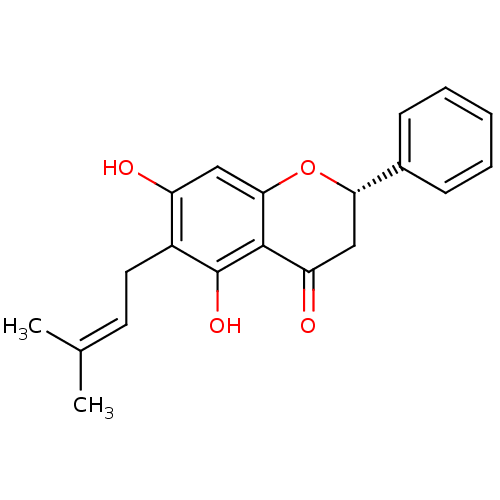 (CHEMBL2165236) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus C2888 AChE using acetylthiocholine iodide as substrate by Ellman's spectroscopic method | J Nat Prod 75: 2012-2015 (2012) Article DOI: 10.1021/np300660y BindingDB Entry DOI: 10.7270/Q2959JNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50394653 (CHEMBL2165235) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus C2888 AChE using acetylthiocholine iodide as substrate by Ellman's spectroscopic method | J Nat Prod 75: 2012-2015 (2012) Article DOI: 10.1021/np300660y BindingDB Entry DOI: 10.7270/Q2959JNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50394655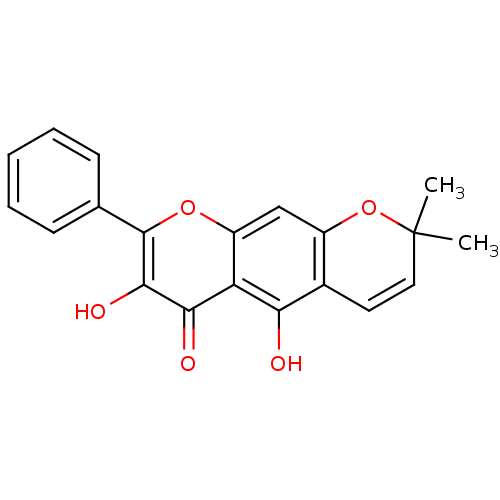 (CHEMBL2165237) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus C2888 AChE using acetylthiocholine iodide as substrate by Ellman's spectroscopic method | J Nat Prod 75: 2012-2015 (2012) Article DOI: 10.1021/np300660y BindingDB Entry DOI: 10.7270/Q2959JNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM26663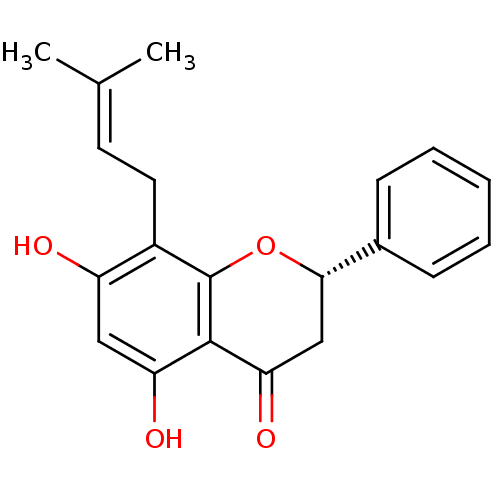 ((2S)-5,7-dihydroxy-8-(3-methylbut-2-en-1-yl)-2-phe...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus C2888 AChE using acetylthiocholine iodide as substrate by Ellman's spectroscopic method | J Nat Prod 75: 2012-2015 (2012) Article DOI: 10.1021/np300660y BindingDB Entry DOI: 10.7270/Q2959JNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50394656 (CHEMBL2165240) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus C2888 AChE using acetylthiocholine iodide as substrate by Ellman's spectroscopic method | J Nat Prod 75: 2012-2015 (2012) Article DOI: 10.1021/np300660y BindingDB Entry DOI: 10.7270/Q2959JNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||