

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292914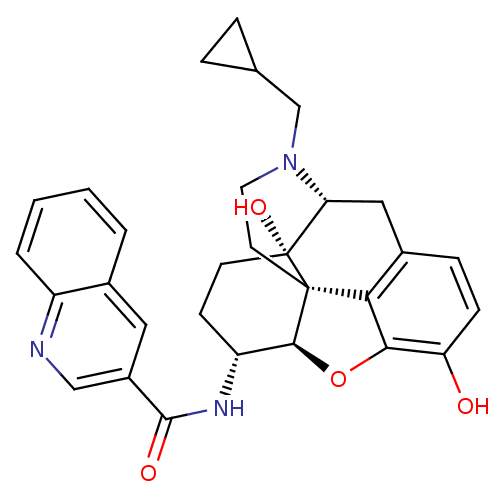 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Activity at monocloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292918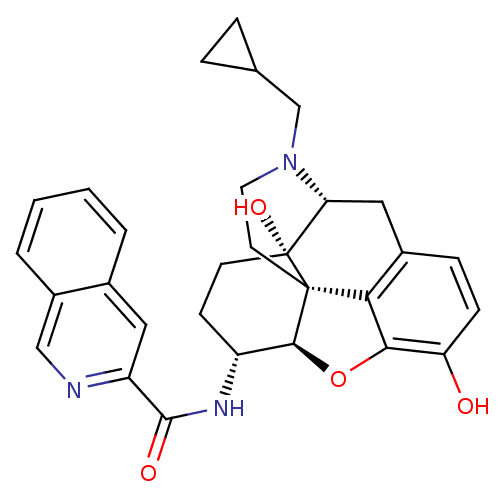 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from monocloned mu opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292916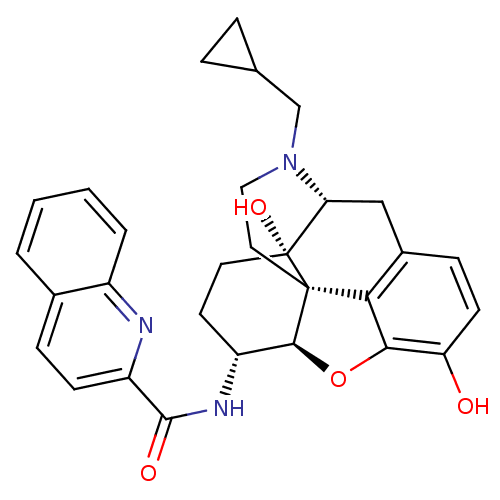 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from monocloned mu opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492288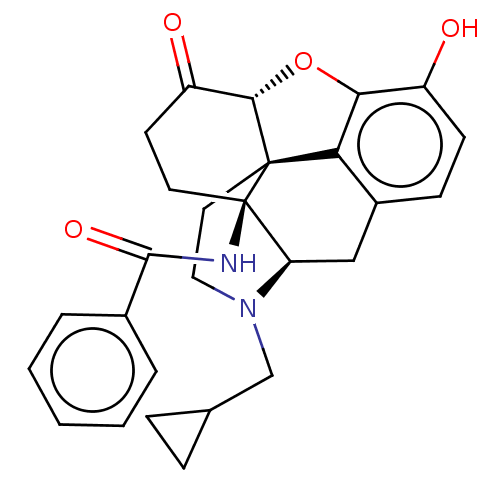 (CHEMBL2397015) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292915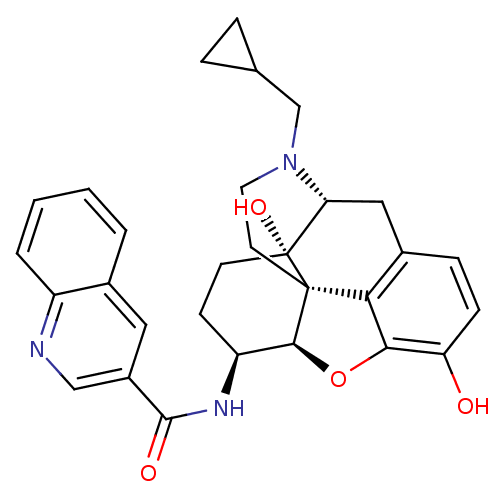 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Activity at monocloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492293 (CHEMBL2397018) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50494375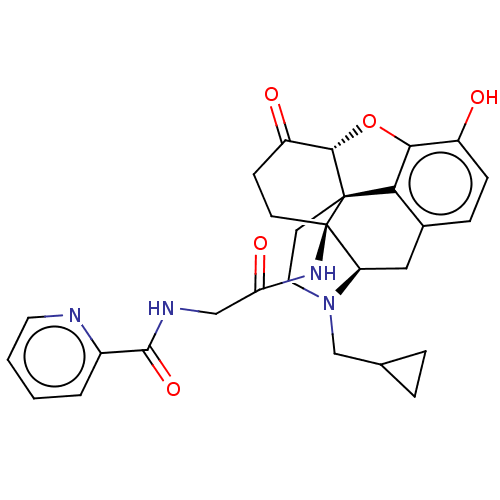 (CHEMBL3086756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266857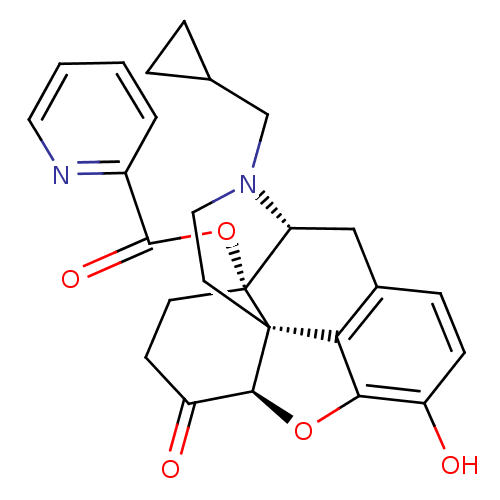 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1825-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.093 BindingDB Entry DOI: 10.7270/Q2GX4CH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266857 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292922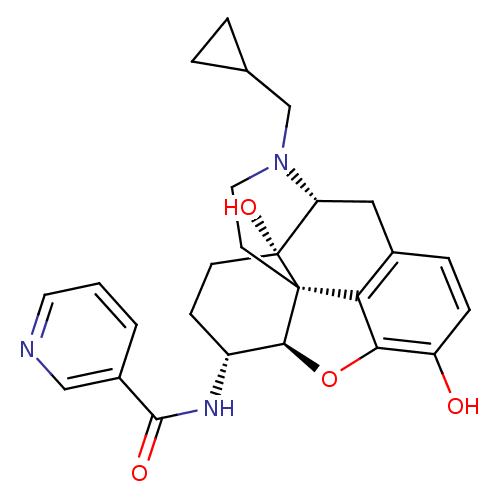 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Activity at monocloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292923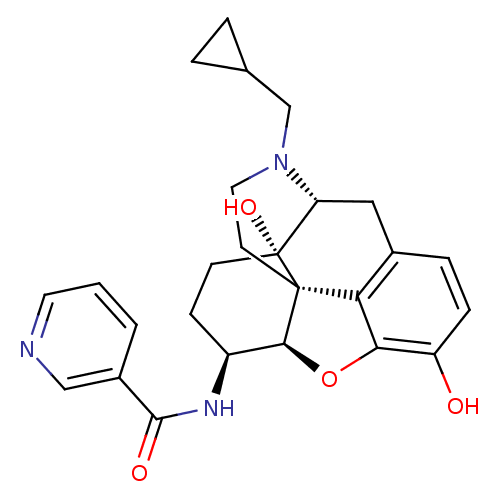 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Activity at monocloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492292 (CHEMBL2397021) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50494377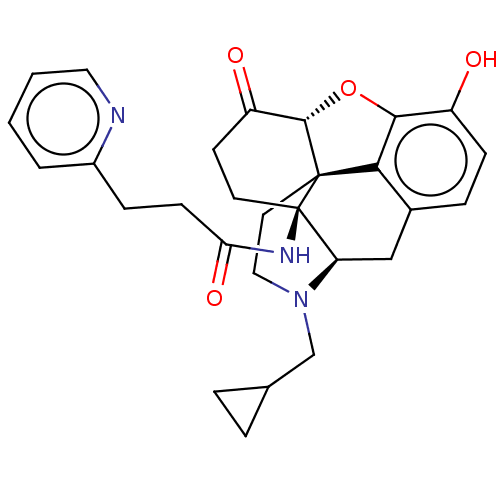 (CHEMBL3086755) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50494376 (CHEMBL3086754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292917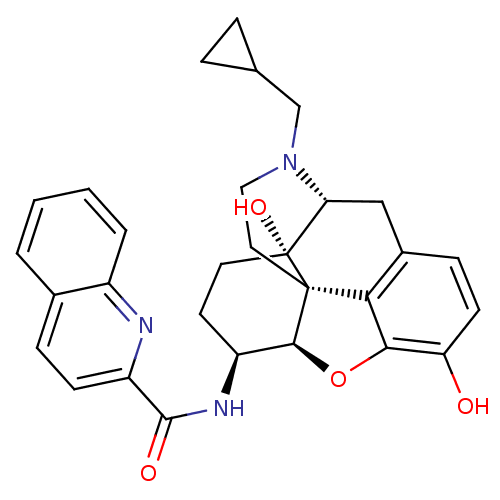 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from monocloned mu opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from monocloned mu opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492287 (CHEMBL2397017) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1825-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.093 BindingDB Entry DOI: 10.7270/Q2GX4CH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492291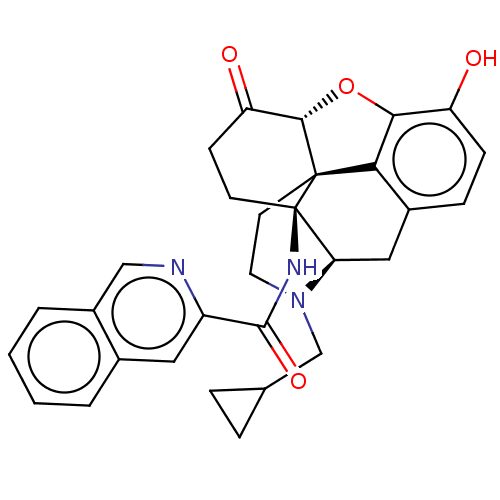 (CHEMBL2397016) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494376 (CHEMBL3086754) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494375 (CHEMBL3086756) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494377 (CHEMBL3086755) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492290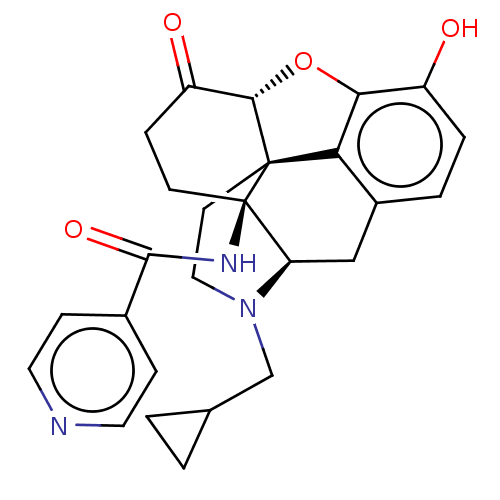 (CHEMBL2397022) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492286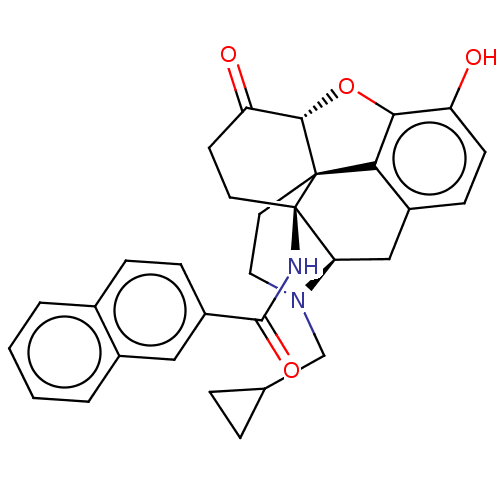 (CHEMBL2397019) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492289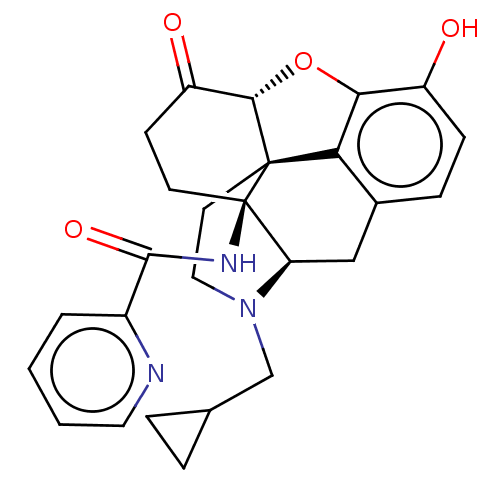 (CHEMBL2397020) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50494375 (CHEMBL3086756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NTI from delta opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292920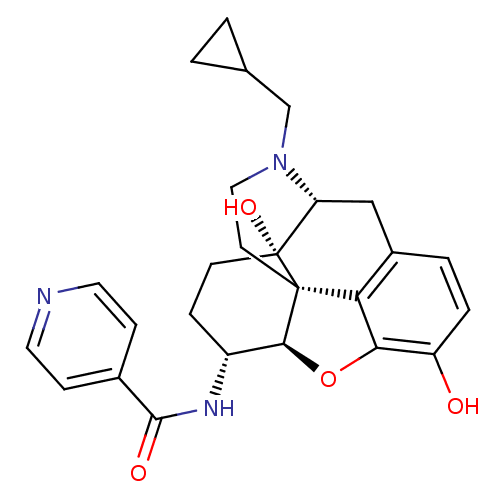 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from monocloned mu opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50247803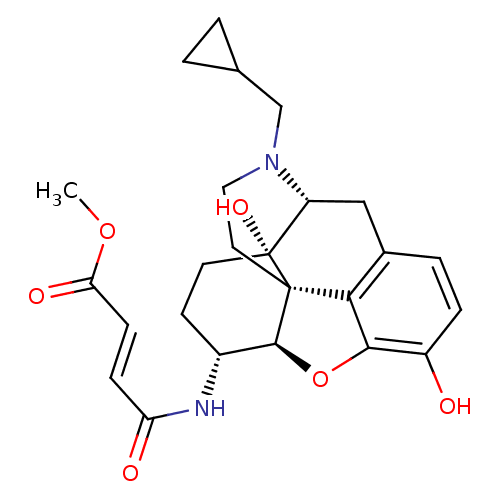 (Beta-Funeltrexamine | CHEMBL473136 | beta-funaltre...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Activity at monocloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50247803 (Beta-Funeltrexamine | CHEMBL473136 | beta-funaltre...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1825-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.093 BindingDB Entry DOI: 10.7270/Q2GX4CH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292921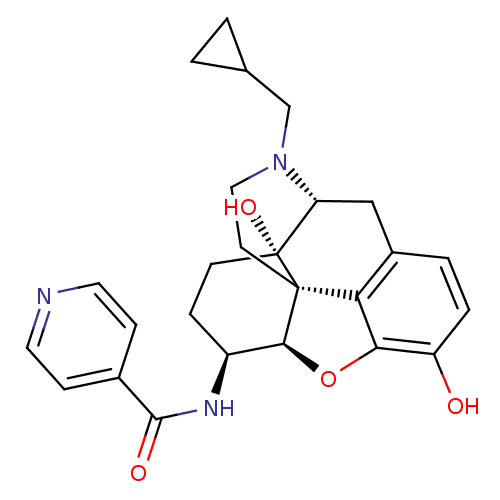 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Activity at monocloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292919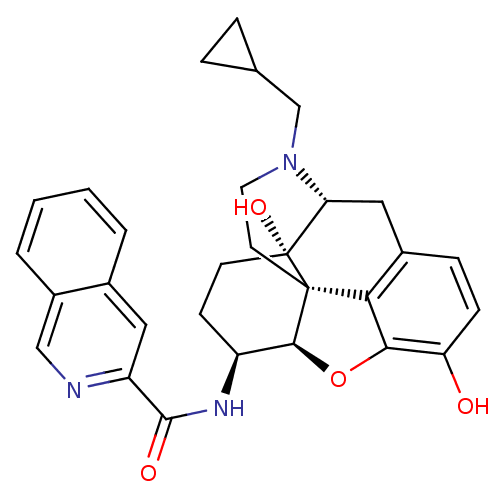 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from monocloned mu opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50292914 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]norBNI from monocloned kappa opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492292 (CHEMBL2397021) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492290 (CHEMBL2397022) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50235942 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Activity at monocloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50247803 (Beta-Funeltrexamine | CHEMBL473136 | beta-funaltre...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]norBNI from monocloned kappa opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50247803 (Beta-Funeltrexamine | CHEMBL473136 | beta-funaltre...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]norBNI from kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1825-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.093 BindingDB Entry DOI: 10.7270/Q2GX4CH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492293 (CHEMBL2397018) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50235940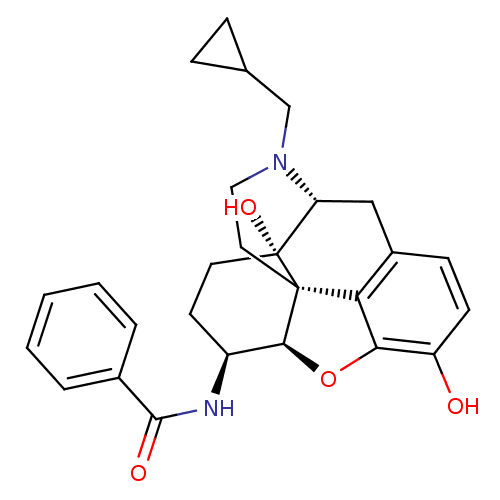 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Activity at monocloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266887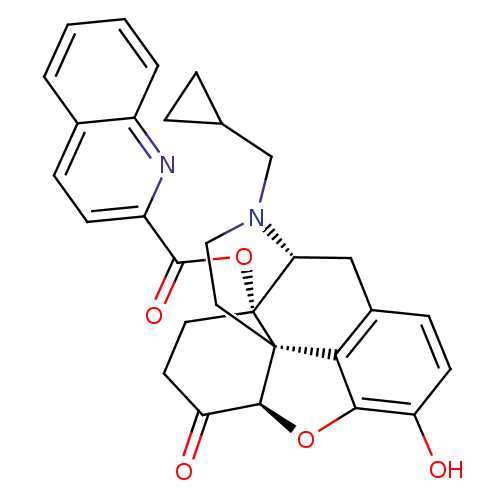 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266887 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1825-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.093 BindingDB Entry DOI: 10.7270/Q2GX4CH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492289 (CHEMBL2397020) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50492293 (CHEMBL2397018) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NTI from delta opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50292918 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]norBNI from monocloned kappa opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266858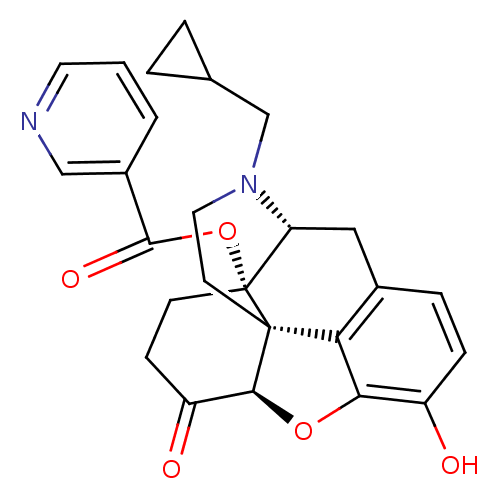 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266858 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1825-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.093 BindingDB Entry DOI: 10.7270/Q2GX4CH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50292915 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]norBNI from monocloned kappa opioid receptor expressed in CHO cells | J Med Chem 52: 1416-27 (2010) Article DOI: 10.1021/jm801272c BindingDB Entry DOI: 10.7270/Q2416XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266827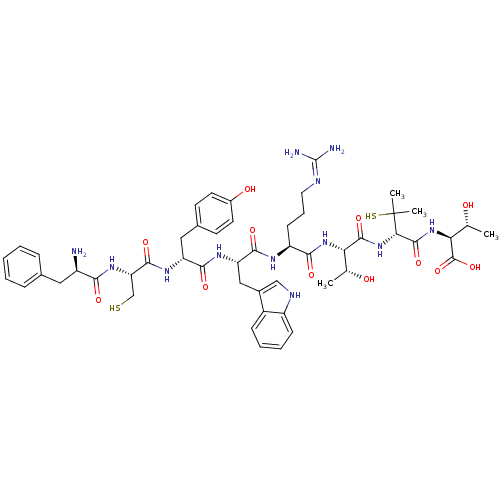 (CHEMBL500766 | D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1825-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.093 BindingDB Entry DOI: 10.7270/Q2GX4CH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 398 total ) | Next | Last >> |