 Found 420 hits with Last Name = 'chen' and Initial = 'lj'
Found 420 hits with Last Name = 'chen' and Initial = 'lj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM9291
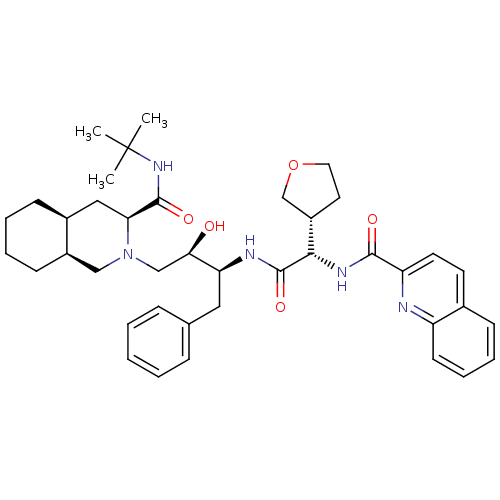
((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)[C@H]1CCOC1 |r| Show InChI InChI=1S/C40H53N5O5/c1-40(2,3)44-38(48)34-22-28-14-7-8-15-29(28)23-45(34)24-35(46)33(21-26-11-5-4-6-12-26)42-39(49)36(30-19-20-50-25-30)43-37(47)32-18-17-27-13-9-10-16-31(27)41-32/h4-6,9-13,16-18,28-30,33-36,46H,7-8,14-15,19-25H2,1-3H3,(H,42,49)(H,43,47)(H,44,48)/t28-,29+,30-,33-,34-,35+,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease was determined in vitro |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM383
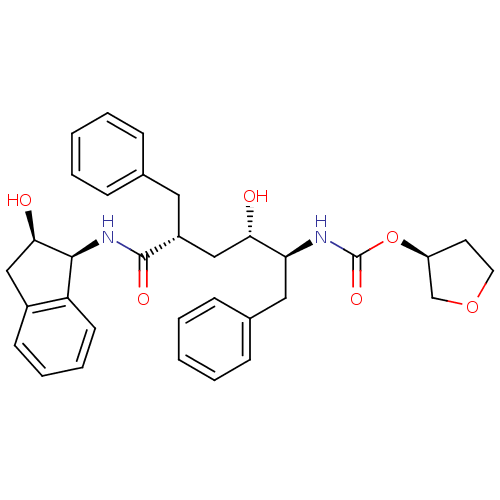
((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1 |r| Show InChI InChI=1S/C33H38N2O6/c36-29(28(18-23-11-5-2-6-12-23)34-33(39)41-26-15-16-40-21-26)20-25(17-22-9-3-1-4-10-22)32(38)35-31-27-14-8-7-13-24(27)19-30(31)37/h1-14,25-26,28-31,36-37H,15-21H2,(H,34,39)(H,35,38)/t25-,26+,28+,29+,30-,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against Human immunodeficiency virus (HIV-1) protease |
Bioorg Med Chem Lett 4: 499-504 (1994)
Article DOI: 10.1016/0960-894X(94)80025-1
BindingDB Entry DOI: 10.7270/Q2GM8777 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM383

((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1 |r| Show InChI InChI=1S/C33H38N2O6/c36-29(28(18-23-11-5-2-6-12-23)34-33(39)41-26-15-16-40-21-26)20-25(17-22-9-3-1-4-10-22)32(38)35-31-27-14-8-7-13-24(27)19-30(31)37/h1-14,25-26,28-31,36-37H,15-21H2,(H,34,39)(H,35,38)/t25-,26+,28+,29+,30-,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease was determined in vitro |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM842

(Benzocycloalkyl Amines deriv. 12 | CHEMBL419923 | ...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)[C@H](O)c2ccccc12 |r| Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(40)34-26(19-22-14-8-5-9-15-22)27(36)20-23(18-21-12-6-4-7-13-21)31(39)35-28-24-16-10-11-17-25(24)29(37)30(28)38/h4-17,23,26-30,36-38H,18-20H2,1-3H3,(H,34,40)(H,35,39)/t23-,26+,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1046
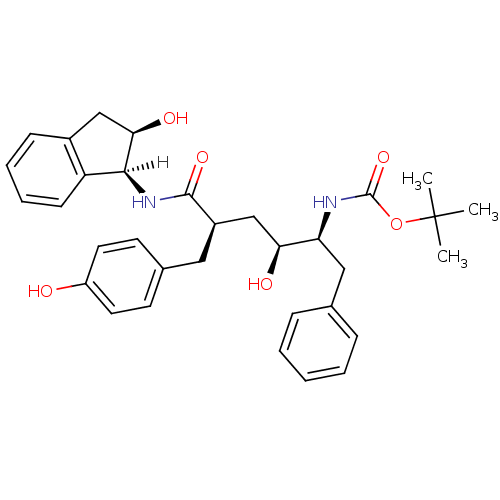
(CHEMBL298316 | L-685,927 | tert-butyl N-[(2S,3S,5R...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(O)cc2)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(40)34-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(36)16-14-22)31(39)35-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,36-38H,17-20H2,1-3H3,(H,34,40)(H,35,39)/t24-,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1039

(CHEMBL297620 | Hydroxyethylene dipeptide isostere ...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(cc2)C(C)(C)C)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C37H48N2O5/c1-36(2,3)28-18-16-25(17-19-28)20-27(34(42)39-33-29-15-11-10-14-26(29)22-32(33)41)23-31(40)30(21-24-12-8-7-9-13-24)38-35(43)44-37(4,5)6/h7-19,27,30-33,40-41H,20-23H2,1-6H3,(H,38,43)(H,39,42)/t27-,30+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50035633
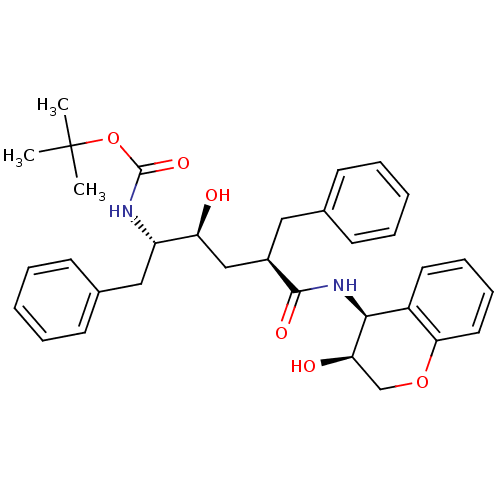
(CHEMBL111143 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)COc2ccccc12 Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(39)34-26(19-23-14-8-5-9-15-23)27(36)20-24(18-22-12-6-4-7-13-22)31(38)35-30-25-16-10-11-17-29(25)40-21-28(30)37/h4-17,24,26-28,30,36-37H,18-21H2,1-3H3,(H,34,39)(H,35,38)/t24-,26+,27+,28-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50035635

(CHEMBL322984 | [(1S,2S,4R)-1-Benzyl-4-((1S,2S,3R)-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H]1[C@H](O)[C@H](O)c2ccccc12 Show InChI InChI=1S/C35H44N2O6/c1-35(2,3)43-34(42)36-28(21-24-15-8-5-9-16-24)29(38)22-25(18-12-17-23-13-6-4-7-14-23)33(41)37-30-26-19-10-11-20-27(26)31(39)32(30)40/h4-11,13-16,19-20,25,28-32,38-40H,12,17-18,21-22H2,1-3H3,(H,36,42)(H,37,41)/t25-,28+,29+,30+,31-,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1031
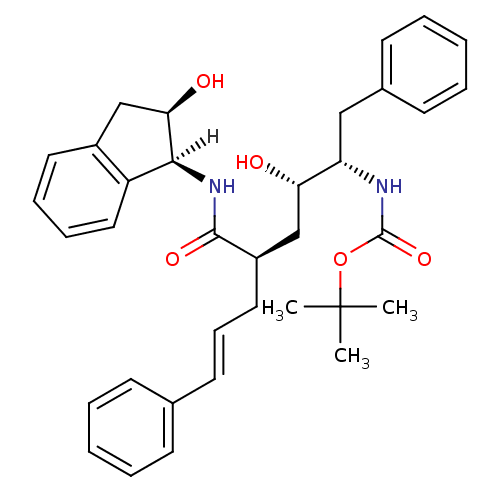
(CHEMBL265514 | Hydroxyethylene dipeptide isostere ...)Show SMILES [H][C@@]1(NC(=O)[C@H](C\C=C\c2ccccc2)C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C35H42N2O5/c1-35(2,3)42-34(41)36-29(21-25-15-8-5-9-16-25)30(38)23-27(19-12-17-24-13-6-4-7-14-24)33(40)37-32-28-20-11-10-18-26(28)22-31(32)39/h4-18,20,27,29-32,38-39H,19,21-23H2,1-3H3,(H,36,41)(H,37,40)/b17-12+/t27-,29+,30+,31-,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease was determined in vitro |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1030
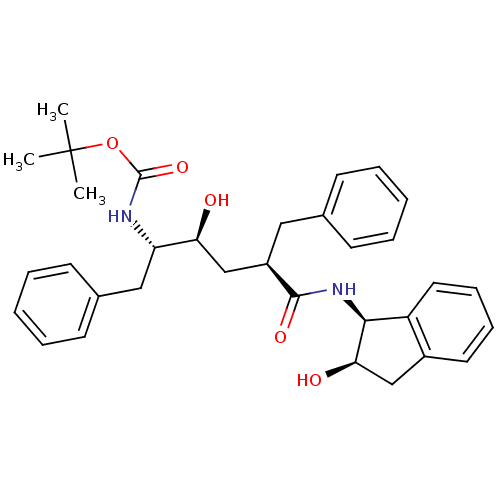
(CHEMBL296115 | L-685,434 | Urethane deriv. 1 | ter...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H40N2O5/c1-33(2,3)40-32(39)34-27(19-23-14-8-5-9-15-23)28(36)21-25(18-22-12-6-4-7-13-22)31(38)35-30-26-17-11-10-16-24(26)20-29(30)37/h4-17,25,27-30,36-37H,18-21H2,1-3H3,(H,34,39)(H,35,38)/t25-,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50035630

(CHEMBL108918 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](CSc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C33H40N2O5S/c1-33(2,3)40-32(39)34-27(18-22-12-6-4-7-13-22)28(36)20-24(21-41-25-15-8-5-9-16-25)31(38)35-30-26-17-11-10-14-23(26)19-29(30)37/h4-17,24,27-30,36-37H,18-21H2,1-3H3,(H,34,39)(H,35,38)/t24-,27-,28-,29+,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1040

(CHEMBL296484 | Hydroxyethylene dipeptide isostere ...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(cc2)C(F)(F)F)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C34H39F3N2O5/c1-33(2,3)44-32(43)38-27(18-21-9-5-4-6-10-21)28(40)20-24(17-22-13-15-25(16-14-22)34(35,36)37)31(42)39-30-26-12-8-7-11-23(26)19-29(30)41/h4-16,24,27-30,40-41H,17-20H2,1-3H3,(H,38,43)(H,39,42)/t24-,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1036

(CHEMBL295904 | Hydroxyethylene dipeptide isostere ...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(cc2)[N+]([O-])=O)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H39N3O7/c1-33(2,3)43-32(40)34-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(16-14-22)36(41)42)31(39)35-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,37-38H,17-20H2,1-3H3,(H,34,40)(H,35,39)/t24-,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1038

(CHEMBL265516 | Hydroxyethylene dipeptide isostere ...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(C)cc2)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C34H42N2O5/c1-22-14-16-24(17-15-22)18-26(32(39)36-31-27-13-9-8-12-25(27)20-30(31)38)21-29(37)28(19-23-10-6-5-7-11-23)35-33(40)41-34(2,3)4/h5-17,26,28-31,37-38H,18-21H2,1-4H3,(H,35,40)(H,36,39)/t26-,28+,29+,30-,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325294

(5-((6-(4-hydroxy-3-methoxyphenyl)-2-oxoindolin-3-y...)Show SMILES COc1cc(ccc1O)-c1ccc2\C(=C\c3cc(C(=O)NCCN4CCCC4)c(C)[se]3)C(=O)Nc2c1 Show InChI InChI=1S/C28H29N3O4Se/c1-17-22(27(33)29-9-12-31-10-3-4-11-31)15-20(36-17)16-23-21-7-5-18(13-24(21)30-28(23)34)19-6-8-25(32)26(14-19)35-2/h5-8,13-16,32H,3-4,9-12H2,1-2H3,(H,29,33)(H,30,34)/b23-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1037
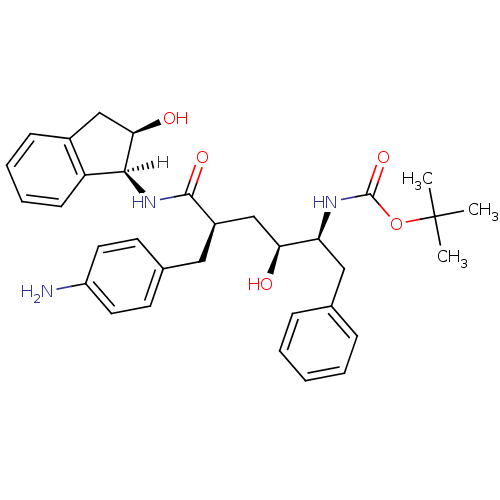
(CHEMBL296493 | Hydroxyethylene dipeptide isostere ...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(N)cc2)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H41N3O5/c1-33(2,3)41-32(40)35-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(34)16-14-22)31(39)36-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,37-38H,17-20,34H2,1-3H3,(H,35,40)(H,36,39)/t24-,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325308

(5-((6-(4-hydroxyphenyl)-2-oxoindolin-3-ylidene)met...)Show SMILES Cc1[se]c(\C=C2/C(=O)Nc3cc(ccc23)-c2ccc(O)cc2)cc1C(=O)NCCN1CCCC1 Show InChI InChI=1S/C27H27N3O3Se/c1-17-23(26(32)28-10-13-30-11-2-3-12-30)15-21(34-17)16-24-22-9-6-19(14-25(22)29-27(24)33)18-4-7-20(31)8-5-18/h4-9,14-16,31H,2-3,10-13H2,1H3,(H,28,32)(H,29,33)/b24-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325307

(CHEMBL1223408 | N-(2-(diethylamino)ethyl)-5-((6-(4...)Show SMILES CCN(CC)CCNC(=O)c1cc(\C=C2/C(=O)Nc3cc(ccc23)-c2ccc(O)cc2)[se]c1C Show InChI InChI=1S/C27H29N3O3Se/c1-4-30(5-2)13-12-28-26(32)23-15-21(34-17(23)3)16-24-22-11-8-19(14-25(22)29-27(24)33)18-6-9-20(31)10-7-18/h6-11,14-16,31H,4-5,12-13H2,1-3H3,(H,28,32)(H,29,33)/b24-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325296
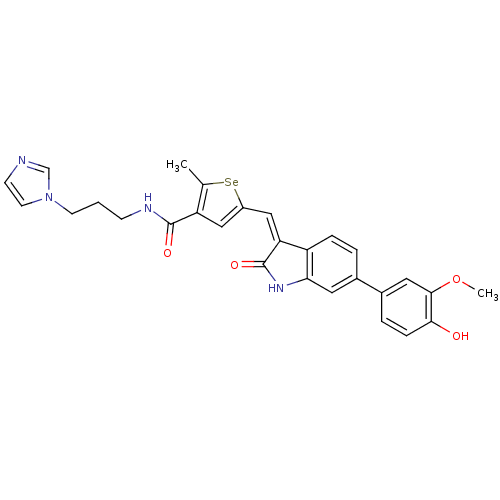
(CHEMBL1223263 | N-(3-(1H-imidazol-1-yl)propyl)-5-(...)Show SMILES COc1cc(ccc1O)-c1ccc2\C(=C\c3cc(C(=O)NCCCn4ccnc4)c(C)[se]3)C(=O)Nc2c1 Show InChI InChI=1S/C28H26N4O4Se/c1-17-22(27(34)30-8-3-10-32-11-9-29-16-32)14-20(37-17)15-23-21-6-4-18(12-24(21)31-28(23)35)19-5-7-25(33)26(13-19)36-2/h4-7,9,11-16,33H,3,8,10H2,1-2H3,(H,30,34)(H,31,35)/b23-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325292

(CHEMBL1223182 | N-(2-(dimethylamino)ethyl)-5-((6-(...)Show SMILES COc1cc(ccc1O)-c1ccc2\C(=C\c3cc(C(=O)NCCN(C)C)c(C)[se]3)C(=O)Nc2c1 Show InChI InChI=1S/C26H27N3O4Se/c1-15-20(25(31)27-9-10-29(2)3)13-18(34-15)14-21-19-7-5-16(11-22(19)28-26(21)32)17-6-8-23(30)24(12-17)33-4/h5-8,11-14,30H,9-10H2,1-4H3,(H,27,31)(H,28,32)/b21-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325310

(CHEMBL1223478 | N-(3-(1H-imidazol-1-yl)propyl)-5-(...)Show SMILES Cc1[se]c(\C=C2/C(=O)Nc3cc(ccc23)-c2ccc(O)cc2)cc1C(=O)NCCCn1ccnc1 Show InChI InChI=1S/C27H24N4O3Se/c1-17-23(26(33)29-9-2-11-31-12-10-28-16-31)14-21(35-17)15-24-22-8-5-19(13-25(22)30-27(24)34)18-3-6-20(32)7-4-18/h3-8,10,12-16,32H,2,9,11H2,1H3,(H,29,33)(H,30,34)/b24-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50035628
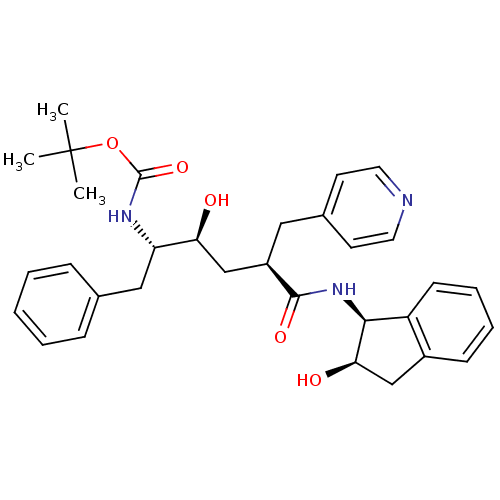
(CHEMBL430692 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccncc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C32H39N3O5/c1-32(2,3)40-31(39)34-26(18-21-9-5-4-6-10-21)27(36)20-24(17-22-13-15-33-16-14-22)30(38)35-29-25-12-8-7-11-23(25)19-28(29)37/h4-16,24,26-29,36-37H,17-20H2,1-3H3,(H,34,39)(H,35,38)/t24-,26+,27+,28-,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325306
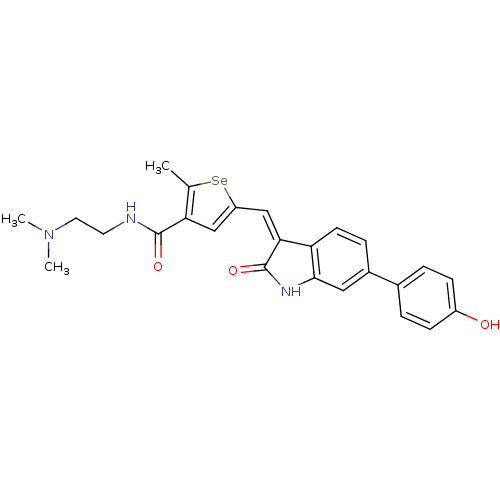
(CHEMBL1223407 | N-(2-(dimethylamino)ethyl)-5-((6-(...)Show SMILES CN(C)CCNC(=O)c1cc(\C=C2/C(=O)Nc3cc(ccc23)-c2ccc(O)cc2)[se]c1C Show InChI InChI=1S/C25H25N3O3Se/c1-15-21(24(30)26-10-11-28(2)3)13-19(32-15)14-22-20-9-6-17(12-23(20)27-25(22)31)16-4-7-18(29)8-5-16/h4-9,12-14,29H,10-11H2,1-3H3,(H,26,30)(H,27,31)/b22-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1041

(CHEMBL47514 | Hydroxyethylene dipeptide isostere 3...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2c(F)c(F)c(F)c(F)c2F)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H35F5N2O5/c1-33(2,3)45-32(44)39-22(13-17-9-5-4-6-10-17)23(41)16-19(14-21-25(34)27(36)29(38)28(37)26(21)35)31(43)40-30-20-12-8-7-11-18(20)15-24(30)42/h4-12,19,22-24,30,41-42H,13-16H2,1-3H3,(H,39,44)(H,40,43)/t19-,22+,23+,24-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325293

(CHEMBL1223183 | N-(2-(diethylamino)ethyl)-5-((6-(4...)Show SMILES CCN(CC)CCNC(=O)c1cc(\C=C2/C(=O)Nc3cc(ccc23)-c2ccc(O)c(OC)c2)[se]c1C Show InChI InChI=1S/C28H31N3O4Se/c1-5-31(6-2)12-11-29-27(33)22-15-20(36-17(22)3)16-23-21-9-7-18(13-24(21)30-28(23)34)19-8-10-25(32)26(14-19)35-4/h7-10,13-16,32H,5-6,11-12H2,1-4H3,(H,29,33)(H,30,34)/b23-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50422052

(CHEMBL109952 | L-700417)Show SMILES OC(C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C39H42N2O5/c42-31(21-29(19-25-11-3-1-4-12-25)38(45)40-36-32-17-9-7-15-27(32)23-34(36)43)22-30(20-26-13-5-2-6-14-26)39(46)41-37-33-18-10-8-16-28(33)24-35(37)44/h1-18,29-31,34-37,42-44H,19-24H2,(H,40,45)(H,41,46)/t29-,30-,34-,35-,36+,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease was determined in vitro |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50035631

(CHEMBL325551 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)CCc2ccccc12 Show InChI InChI=1S/C34H42N2O5/c1-34(2,3)41-33(40)35-28(21-24-14-8-5-9-15-24)30(38)22-26(20-23-12-6-4-7-13-23)32(39)36-31-27-17-11-10-16-25(27)18-19-29(31)37/h4-17,26,28-31,37-38H,18-22H2,1-3H3,(H,35,40)(H,36,39)/t26-,28+,29-,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1035

(CHEMBL296855 | Hydroxyethylene dipeptide isostere ...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(I)cc2)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H39IN2O5/c1-33(2,3)41-32(40)35-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(34)16-14-22)31(39)36-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,37-38H,17-20H2,1-3H3,(H,35,40)(H,36,39)/t24-,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325309

(5-((6-(4-hydroxyphenyl)-2-oxoindolin-3-ylidene)met...)Show SMILES Cc1[se]c(\C=C2/C(=O)Nc3cc(ccc23)-c2ccc(O)cc2)cc1C(=O)NCCN1CCCCO1 Show InChI InChI=1S/C27H27N3O4Se/c1-17-23(26(32)28-10-12-30-11-2-3-13-34-30)15-21(35-17)16-24-22-9-6-19(14-25(22)29-27(24)33)18-4-7-20(31)8-5-18/h4-9,14-16,31H,2-3,10-13H2,1H3,(H,28,32)(H,29,33)/b24-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Yes |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086454
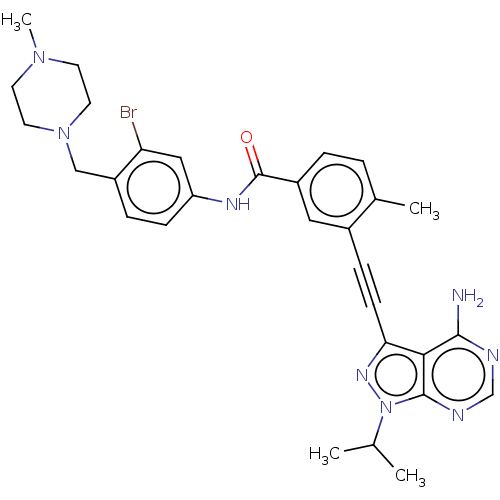
(CHEMBL3425518)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Br)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33BrN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325295

(5-((6-(4-hydroxy-3-methoxyphenyl)-2-oxoindolin-3-y...)Show SMILES COc1cc(ccc1O)-c1ccc2\C(=C\c3cc(C(=O)NCCN4CCOCC4)c(C)[se]3)C(=O)Nc2c1 Show InChI InChI=1S/C28H29N3O5Se/c1-17-22(27(33)29-7-8-31-9-11-36-12-10-31)15-20(37-17)16-23-21-5-3-18(13-24(21)30-28(23)34)19-4-6-25(32)26(14-19)35-2/h3-6,13-16,32H,7-12H2,1-2H3,(H,29,33)(H,30,34)/b23-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50422053

(CHEMBL322037)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)\C=C(\Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C33H38N2O5/c1-33(2,3)40-32(39)34-27(19-23-14-8-5-9-15-23)28(36)21-25(18-22-12-6-4-7-13-22)31(38)35-30-26-17-11-10-16-24(26)20-29(30)37/h4-17,21,27-30,36-37H,18-20H2,1-3H3,(H,34,39)(H,35,38)/b25-21-/t27-,28-,29+,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease was determined in vitro |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283953

(CHEMBL357039 | {(1S,2S)-1-Benzyl-3-[(S)-3-benzyl-1...)Show SMILES O[C@@H](C[C@]1(Cc2ccccc2)CCN([C@@H]2CS(=O)(=O)Cc3ccccc23)C1=O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1 Show InChI InChI=1S/C35H40N2O7S/c38-32(30(19-25-9-3-1-4-10-25)36-34(40)44-28-15-18-43-22-28)21-35(20-26-11-5-2-6-12-26)16-17-37(33(35)39)31-24-45(41,42)23-27-13-7-8-14-29(27)31/h1-14,28,30-32,38H,15-24H2,(H,36,40)/t28-,30-,31+,32-,35+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against Human immunodeficiency virus (HIV-1) protease |
Bioorg Med Chem Lett 4: 499-504 (1994)
Article DOI: 10.1016/0960-894X(94)80025-1
BindingDB Entry DOI: 10.7270/Q2GM8777 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086457
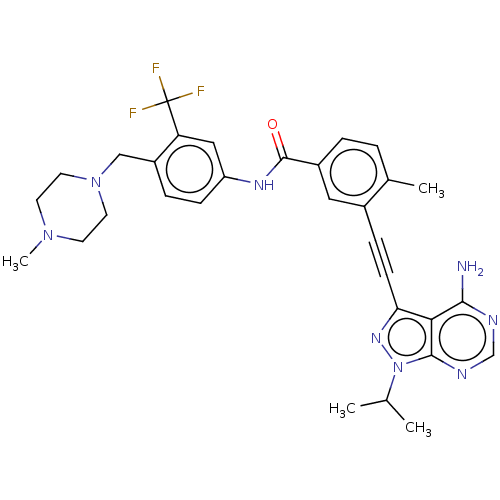
(CHEMBL3426217)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C31H33F3N8O/c1-19(2)42-29-27(28(35)36-18-37-29)26(39-42)10-8-21-15-22(6-5-20(21)3)30(43)38-24-9-7-23(25(16-24)31(32,33)34)17-41-13-11-40(4)12-14-41/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,38,43)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086451
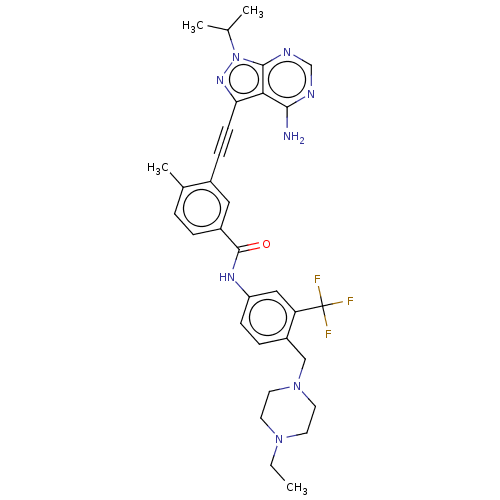
(CHEMBL3426222)Show SMILES CCN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C(C)C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H35F3N8O/c1-5-41-12-14-42(15-13-41)18-24-8-10-25(17-26(24)32(33,34)35)39-31(44)23-7-6-21(4)22(16-23)9-11-27-28-29(36)37-19-38-30(28)43(40-27)20(2)3/h6-8,10,16-17,19-20H,5,12-15,18H2,1-4H3,(H,39,44)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086455
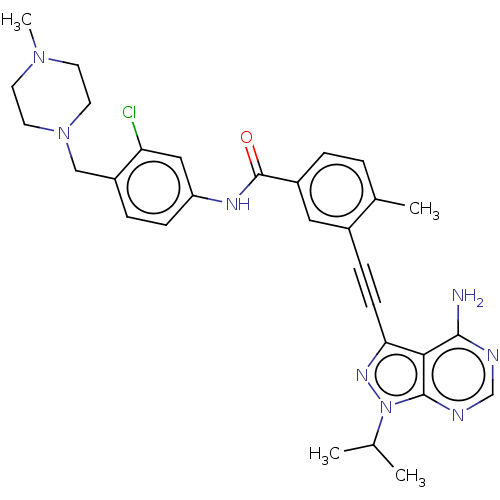
(CHEMBL3426219)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Cl)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33ClN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325287

(CHEMBL1223044 | N-(2-(dimethylamino)ethyl)-5-((6-(...)Show SMILES COc1cc(ccc1O)-c1ccc2\C(=C\c3ccc([se]3)C(=O)NCCN(C)C)C(=O)Nc2c1 Show InChI InChI=1S/C25H25N3O4Se/c1-28(2)11-10-26-25(31)23-9-6-17(33-23)14-19-18-7-4-15(12-20(18)27-24(19)30)16-5-8-21(29)22(13-16)32-3/h4-9,12-14,29H,10-11H2,1-3H3,(H,26,31)(H,27,30)/b19-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086453

(CHEMBL3426220 | US10266537, Compound 14)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2cccc(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C25H21F3N6O/c1-14(2)34-23-21(22(29)30-13-31-23)20(33-34)10-9-16-11-17(8-7-15(16)3)24(35)32-19-6-4-5-18(12-19)25(26,27)28/h4-8,11-14H,1-3H3,(H,32,35)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325291

(CHEMBL1223118 | N-(3-(1H-imidazol-1-yl)propyl)-5-(...)Show SMILES COc1cc(ccc1O)-c1ccc2\C(=C\c3ccc([se]3)C(=O)NCCCn3ccnc3)C(=O)Nc2c1 Show InChI InChI=1S/C27H24N4O4Se/c1-35-24-14-18(4-7-23(24)32)17-3-6-20-21(26(33)30-22(20)13-17)15-19-5-8-25(36-19)27(34)29-9-2-11-31-12-10-28-16-31/h3-8,10,12-16,32H,2,9,11H2,1H3,(H,29,34)(H,30,33)/b21-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086450
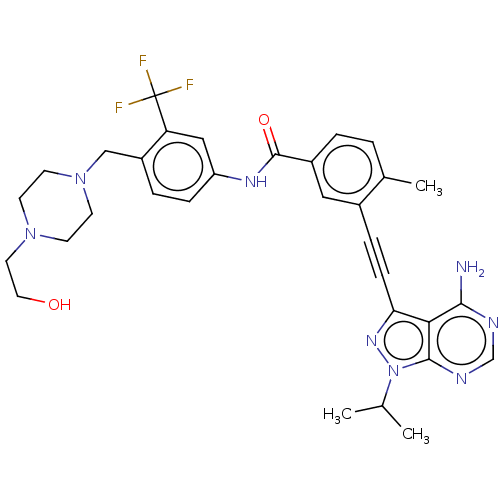
(CHEMBL3426223)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C32H35F3N8O2/c1-20(2)43-30-28(29(36)37-19-38-30)27(40-43)9-7-22-16-23(5-4-21(22)3)31(45)39-25-8-6-24(26(17-25)32(33,34)35)18-42-12-10-41(11-13-42)14-15-44/h4-6,8,16-17,19-20,44H,10-15,18H2,1-3H3,(H,39,45)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086456

(CHEMBL3426218 | US10266537, Compound 17)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C30H34N8O/c1-20(2)38-29-27(28(31)32-19-33-29)26(35-38)12-9-23-17-24(8-5-21(23)3)30(39)34-25-10-6-22(7-11-25)18-37-15-13-36(4)14-16-37/h5-8,10-11,17,19-20H,13-16,18H2,1-4H3,(H,34,39)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325304
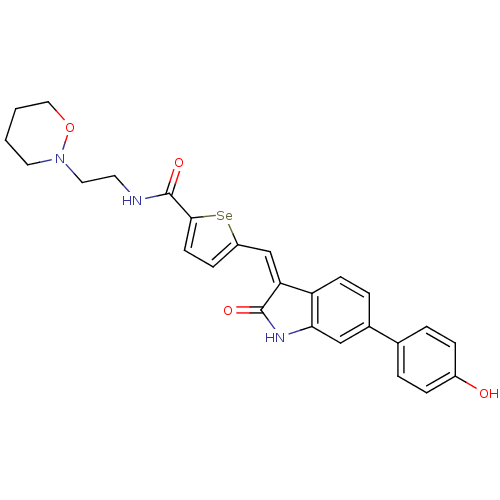
(5-((6-(4-hydroxyphenyl)-2-oxoindolin-3-ylidene)met...)Show SMILES Oc1ccc(cc1)-c1ccc2\C(=C\c3ccc([se]3)C(=O)NCCN3CCCCO3)C(=O)Nc2c1 Show InChI InChI=1S/C26H25N3O4Se/c30-19-6-3-17(4-7-19)18-5-9-21-22(25(31)28-23(21)15-18)16-20-8-10-24(34-20)26(32)27-11-13-29-12-1-2-14-33-29/h3-10,15-16,30H,1-2,11-14H2,(H,27,32)(H,28,31)/b22-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325289
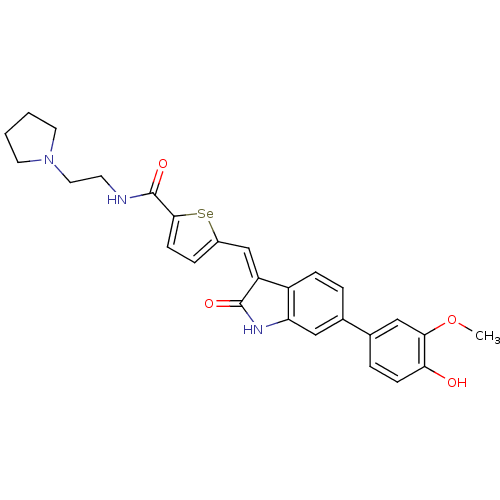
(5-((6-(4-hydroxy-3-methoxyphenyl)-2-oxoindolin-3-y...)Show SMILES COc1cc(ccc1O)-c1ccc2\C(=C\c3ccc([se]3)C(=O)NCCN3CCCC3)C(=O)Nc2c1 Show InChI InChI=1S/C27H27N3O4Se/c1-34-24-15-18(5-8-23(24)31)17-4-7-20-21(26(32)29-22(20)14-17)16-19-6-9-25(35-19)27(33)28-10-13-30-11-2-3-12-30/h4-9,14-16,31H,2-3,10-13H2,1H3,(H,28,33)(H,29,32)/b21-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325302

(CHEMBL1223338 | N-(2-(diethylamino)ethyl)-5-((6-(4...)Show SMILES CCN(CC)CCNC(=O)c1ccc(\C=C2/C(=O)Nc3cc(ccc23)-c2ccc(O)cc2)[se]1 Show InChI InChI=1S/C26H27N3O3Se/c1-3-29(4-2)14-13-27-26(32)24-12-10-20(33-24)16-22-21-11-7-18(15-23(21)28-25(22)31)17-5-8-19(30)9-6-17/h5-12,15-16,30H,3-4,13-14H2,1-2H3,(H,27,32)(H,28,31)/b22-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Chk1after 30 mins |
Bioorg Med Chem Lett 20: 5065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.034
BindingDB Entry DOI: 10.7270/Q2NZ87TV |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086586

(CHEMBL3426234 | US10266537, Compound 29)Show SMILES CCn1nc(C#Cc2cccc(c2)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C29H29F3N8O/c1-3-40-27-25(26(33)34-18-35-27)24(37-40)10-7-19-5-4-6-20(15-19)28(41)36-22-9-8-21(23(16-22)29(30,31)32)17-39-13-11-38(2)12-14-39/h4-6,8-9,15-16,18H,3,11-14,17H2,1-2H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data