

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448144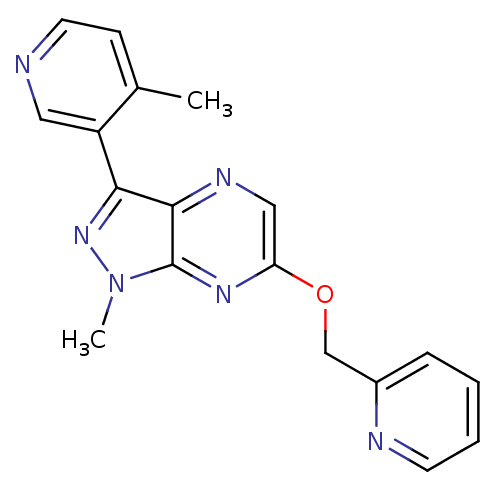 (CHEMBL3122212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240887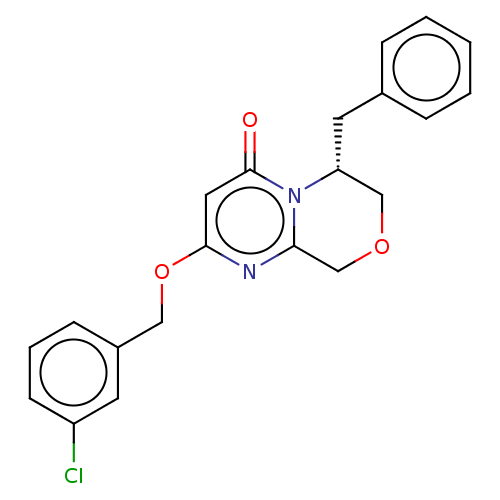 (CHEMBL4066731) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002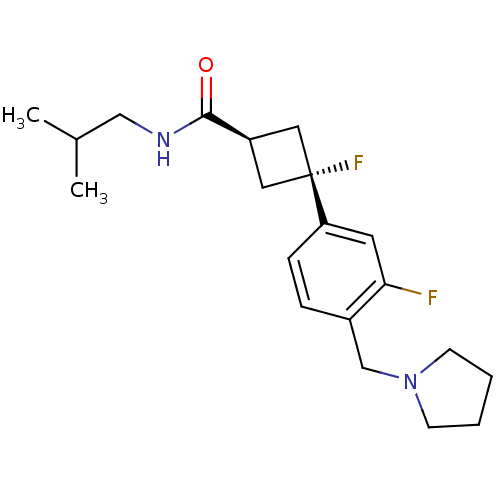 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448157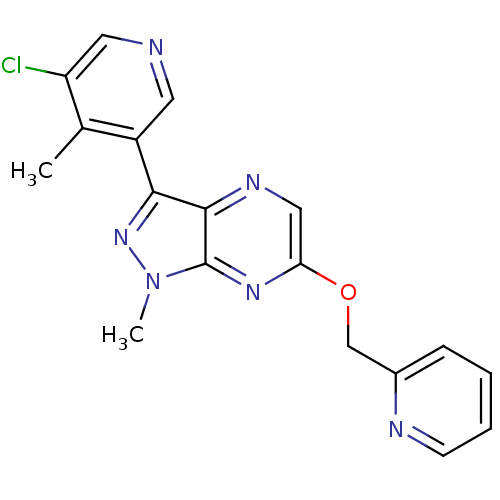 (CHEMBL3122215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401007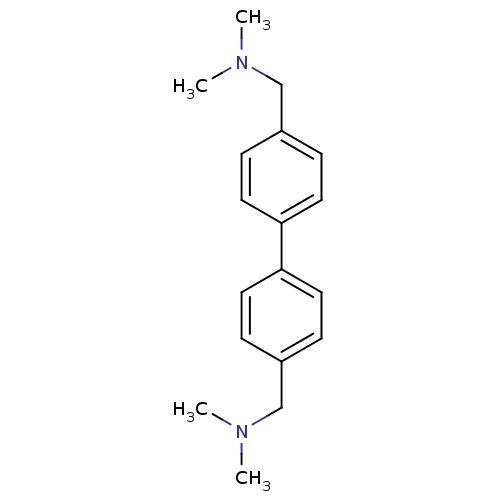 (CHEMBL209478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401007 (CHEMBL209478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240900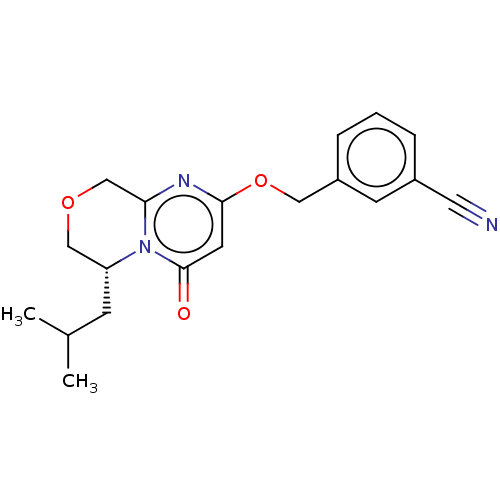 (CHEMBL4091620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003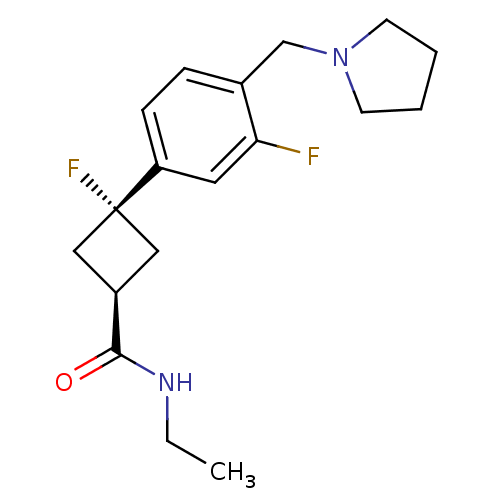 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448161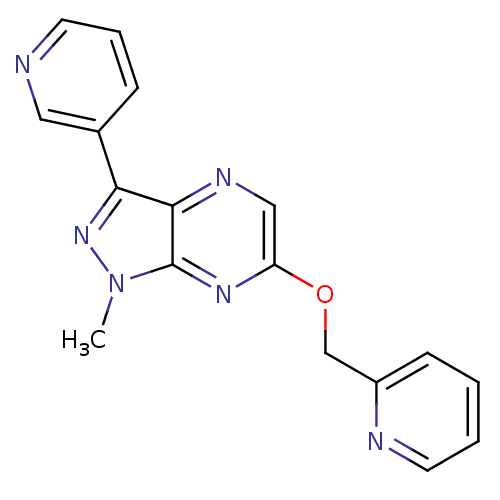 (CHEMBL3122210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448159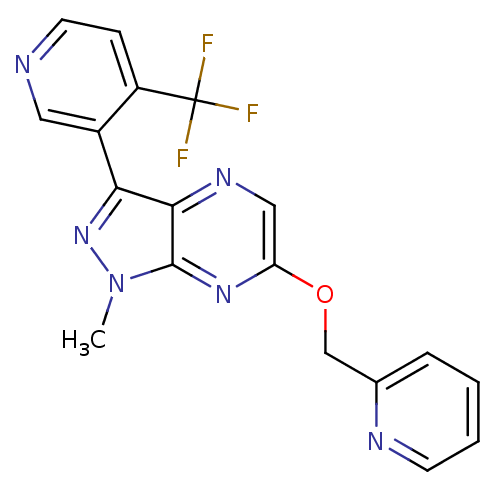 (CHEMBL3122213) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240888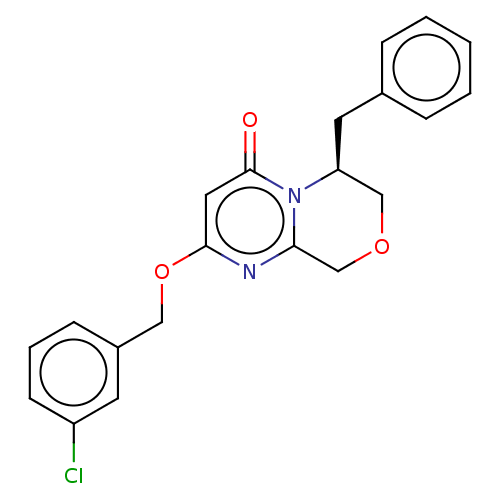 (CHEMBL4094256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240881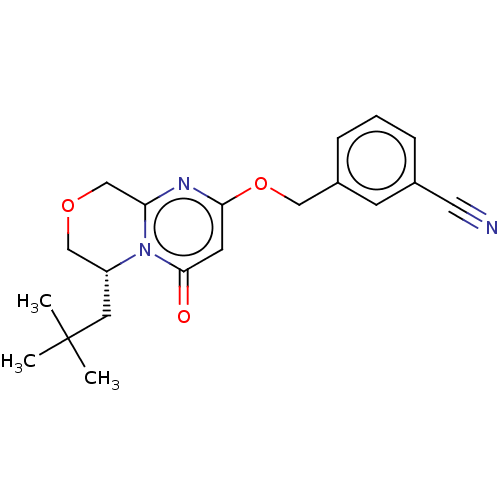 (CHEMBL4090712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401005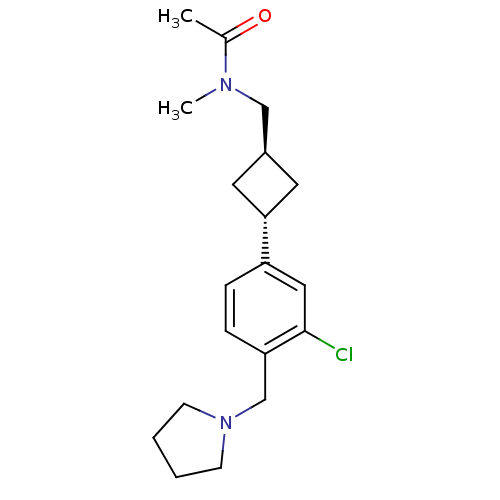 (CHEMBL2206290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401005 (CHEMBL2206290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240885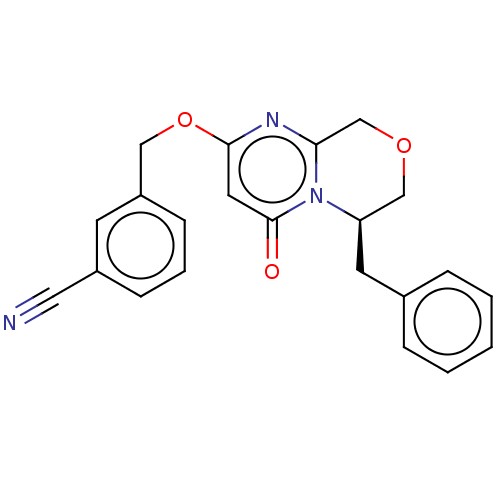 (CHEMBL4064010) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240890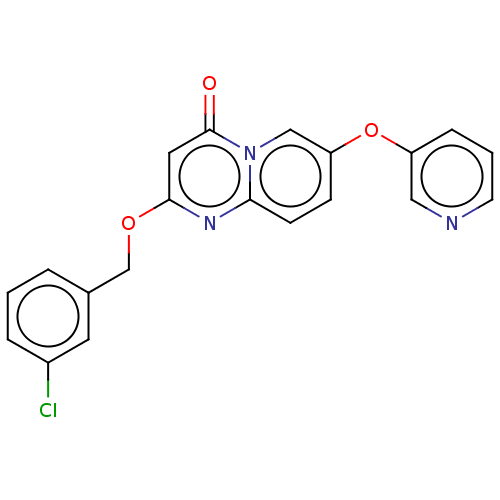 (CHEMBL4078647) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448149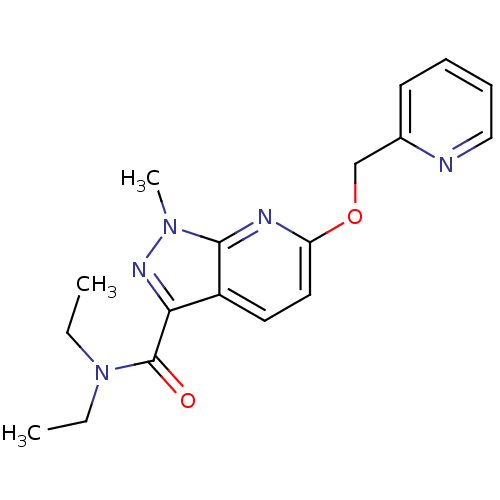 (CHEMBL3122224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448163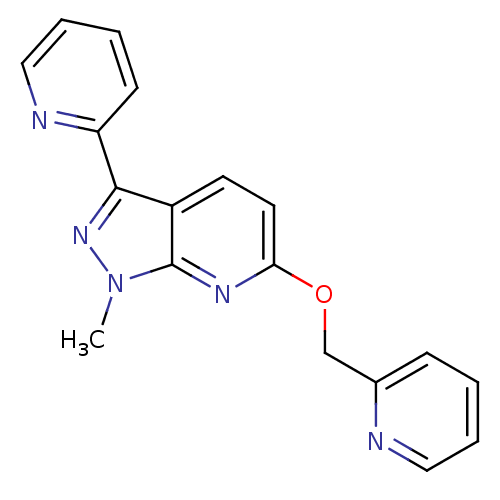 (CHEMBL3122208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240884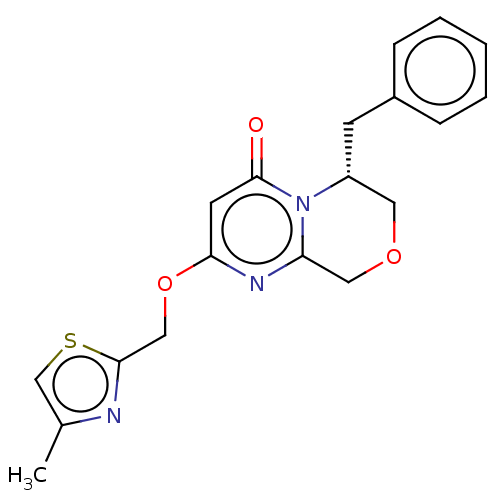 (CHEMBL4085572) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240882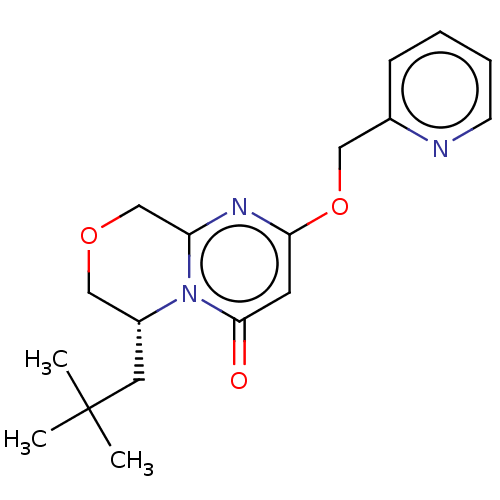 (CHEMBL4063837) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448162 (CHEMBL3122209) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448158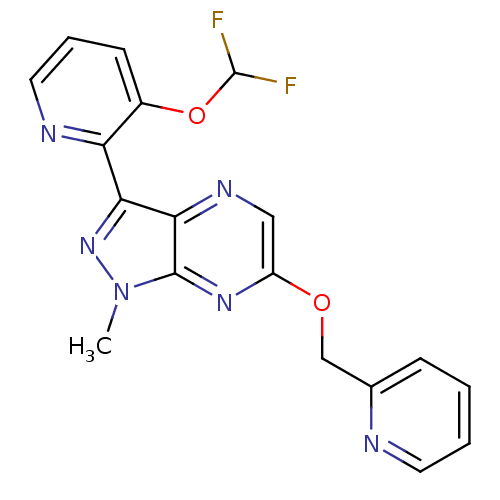 (CHEMBL3122214) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448150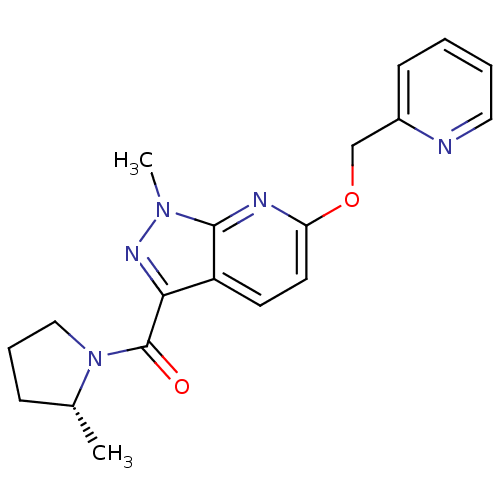 (CHEMBL3122225) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448154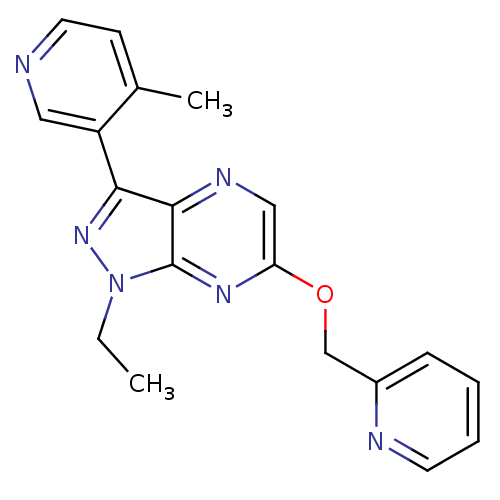 (CHEMBL3122218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240879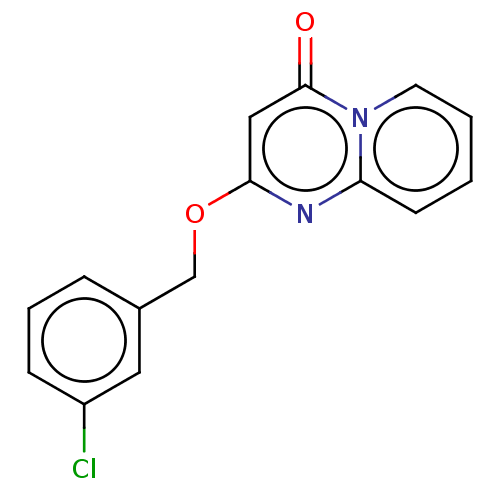 (CHEMBL4089073) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from rat histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from rat histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448148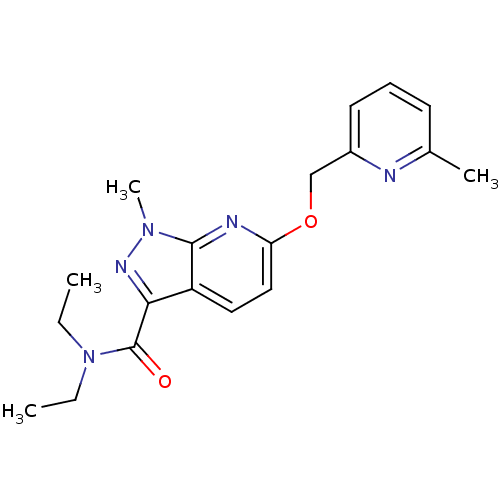 (CHEMBL3122223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448155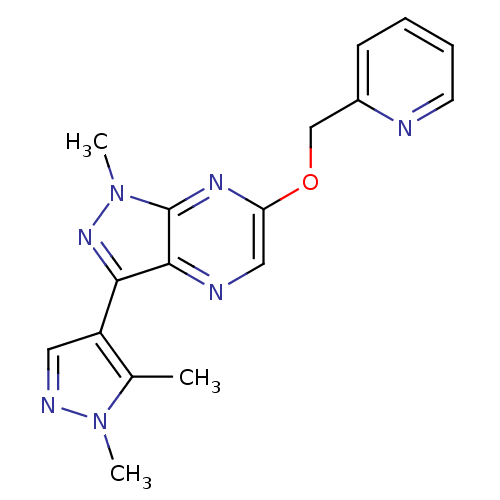 (CHEMBL3122217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401006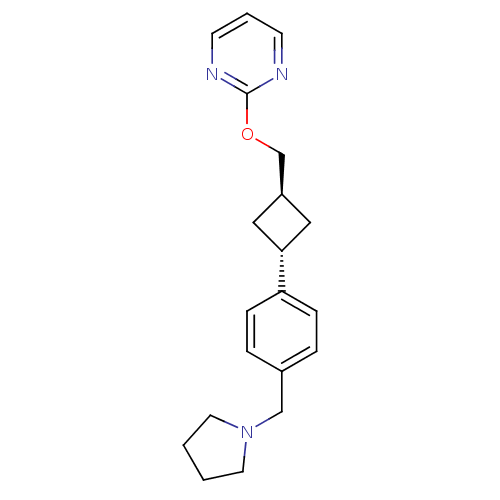 (CHEMBL2206289) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401006 (CHEMBL2206289) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 163 total ) | Next | Last >> |