

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50005118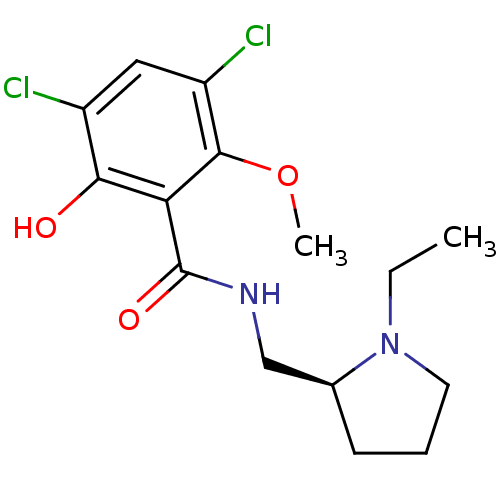 ((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50012967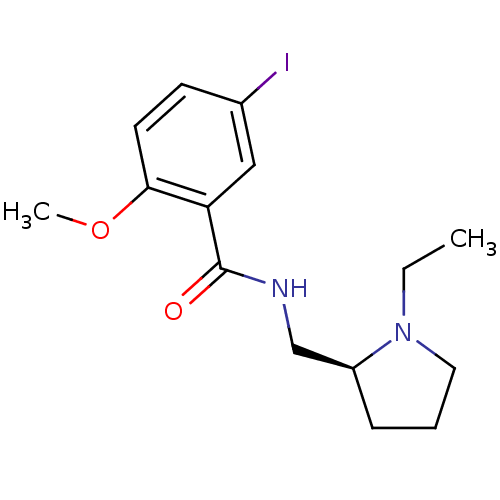 (CHEMBL49897 | N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50012967 (CHEMBL49897 | N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of [3H](S)-sulpiride binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of [3H](S)-sulpiride binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50005118 ((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM11638 (CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM11638 (CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of [3H](S)-sulpiride binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50452484 (CHEMBL2115023) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50452484 (CHEMBL2115023) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of [3H](S)-sulpiride binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of [125I]- (S)-N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-iodo-2-methoxy-benzamide binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of [3H](S)-sulpiride binding in striatal homogenates of rat brain | J Med Chem 31: 2027-33 (1988) BindingDB Entry DOI: 10.7270/Q2FX7B0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||