 Found 65 hits with Last Name = 'dassonville' and Initial = 'a'
Found 65 hits with Last Name = 'dassonville' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50213416
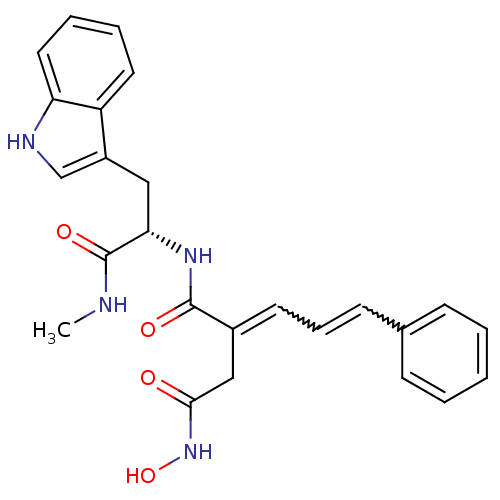
((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50213418
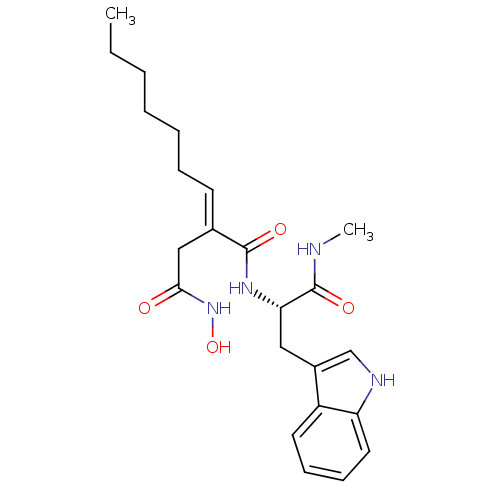
((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP8 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50213416

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50213418

((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50213418

((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50240798
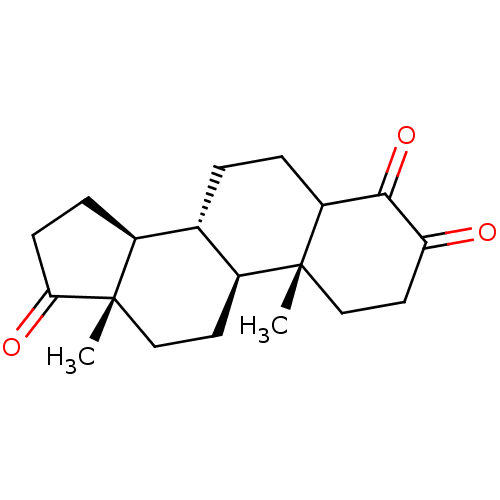
((8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4C(=O)C(=O)CC[C@]34C)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H26O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-14H,3-10H2,1-2H3/t11-,12-,13-,14?,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50213417

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-but-2-en-1-yli...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC=CC)=CC(=O)NO |w:23.25,21.23| Show InChI InChI=1S/C20H24N4O4/c1-3-4-7-13(11-18(25)24-28)19(26)23-17(20(27)21-2)10-14-12-22-16-9-6-5-8-15(14)16/h3-6,8-9,11-12,17,22,28H,7,10H2,1-2H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 913 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50213417

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-but-2-en-1-yli...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC=CC)=CC(=O)NO |w:23.25,21.23| Show InChI InChI=1S/C20H24N4O4/c1-3-4-7-13(11-18(25)24-28)19(26)23-17(20(27)21-2)10-14-12-22-16-9-6-5-8-15(14)16/h3-6,8-9,11-12,17,22,28H,7,10H2,1-2H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 974 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50213417

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-but-2-en-1-yli...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC=CC)=CC(=O)NO |w:23.25,21.23| Show InChI InChI=1S/C20H24N4O4/c1-3-4-7-13(11-18(25)24-28)19(26)23-17(20(27)21-2)10-14-12-22-16-9-6-5-8-15(14)16/h3-6,8-9,11-12,17,22,28H,7,10H2,1-2H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 984 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50213416

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50213416

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50213418

((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602550

(CHEMBL5177406)Show SMILES CCCCCNC[C@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602553

(CHEMBL5204309)Show SMILES CCCCCCCNC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50213417

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-but-2-en-1-yli...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC=CC)=CC(=O)NO |w:23.25,21.23| Show InChI InChI=1S/C20H24N4O4/c1-3-4-7-13(11-18(25)24-28)19(26)23-17(20(27)21-2)10-14-12-22-16-9-6-5-8-15(14)16/h3-6,8-9,11-12,17,22,28H,7,10H2,1-2H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602551

(CHEMBL5181317)Show SMILES CCCCCNC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50213416

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602552
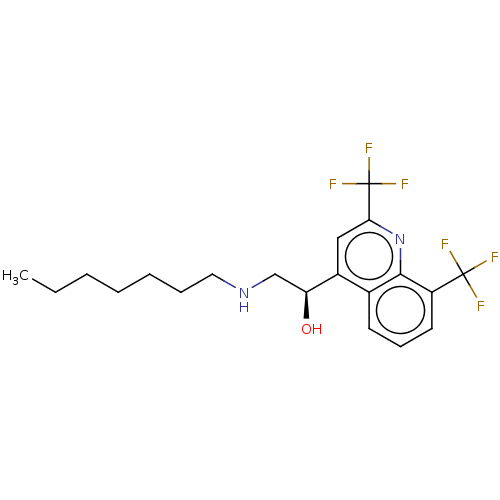
(CHEMBL5191501)Show SMILES CCCCCCCNC[C@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50213418

((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data