 Found 238 hits with Last Name = 'deeks' and Initial = 'n'
Found 238 hits with Last Name = 'deeks' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475465
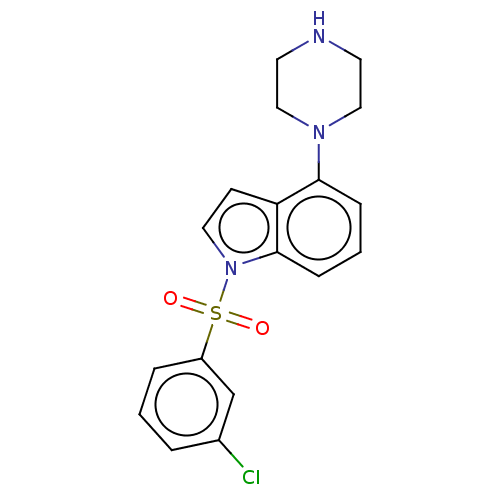
(CHEMBL196410)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-3-1-4-15(13-14)25(23,24)22-10-7-16-17(5-2-6-18(16)22)21-11-8-20-9-12-21/h1-7,10,13,20H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475462
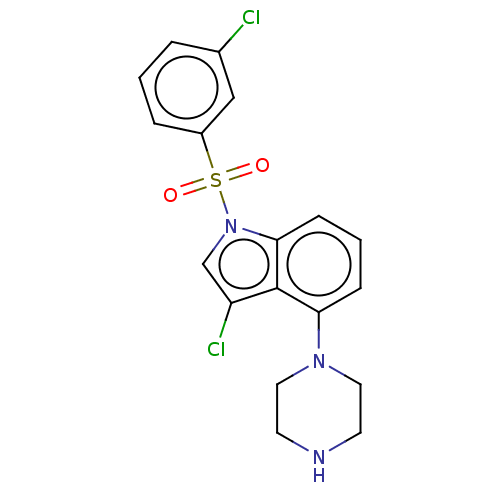
(CHEMBL371375)Show SMILES Clc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-3-1-4-14(11-13)26(24,25)23-12-15(20)18-16(5-2-6-17(18)23)22-9-7-21-8-10-22/h1-6,11-12,21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475480
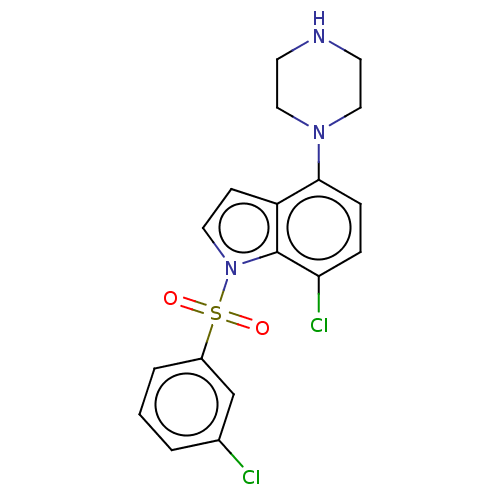
(CHEMBL193629)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(ccc(Cl)c12)N1CCNCC1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(5-4-16(20)18(15)23)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475467

(CHEMBL425015)Show SMILES Clc1cccc(c1)S(=O)(=O)c1c[nH]c2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-13-3-1-4-14(11-13)25(23,24)17-12-21-18-15(17)5-2-6-16(18)22-9-7-20-8-10-22/h1-6,11-12,20-21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475477
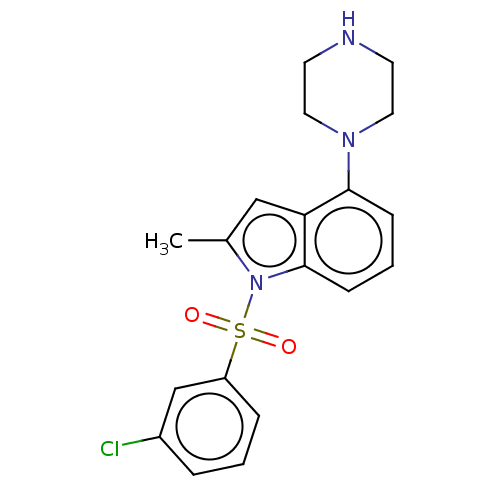
(CHEMBL372929)Show SMILES Cc1cc2c(cccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-12-17-18(22-10-8-21-9-11-22)6-3-7-19(17)23(14)26(24,25)16-5-2-4-15(20)13-16/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475475
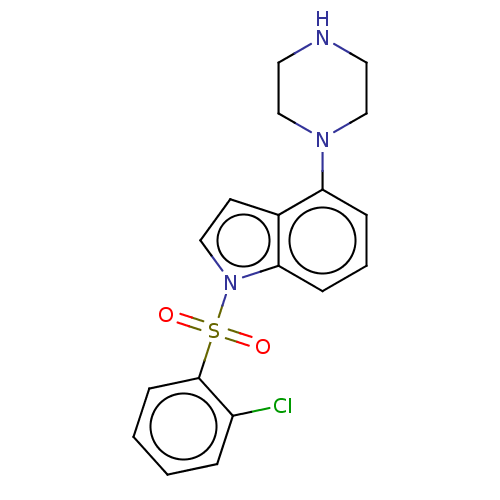
(CHEMBL372513)Show InChI InChI=1S/C18H18ClN3O2S/c19-15-4-1-2-7-18(15)25(23,24)22-11-8-14-16(5-3-6-17(14)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044607
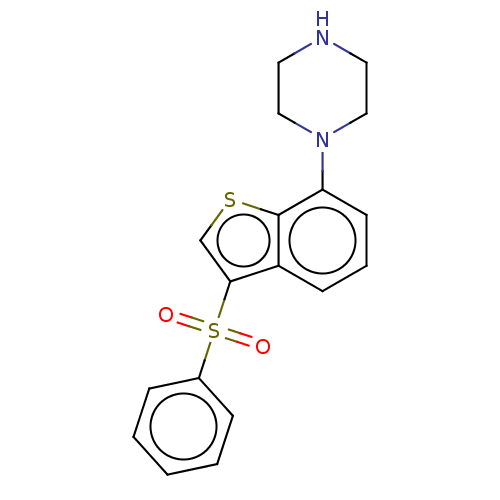
(CHEMBL372537)Show InChI InChI=1S/C18H18N2O2S2/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475463
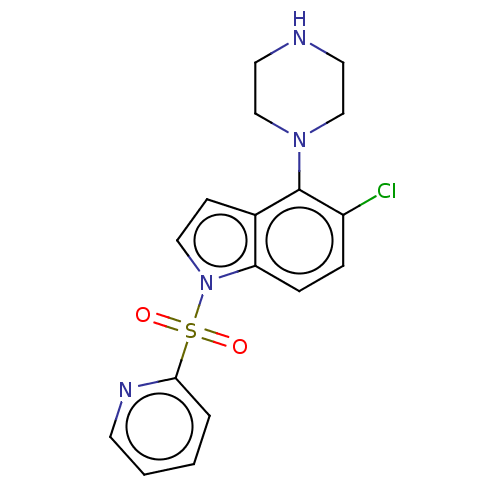
(CHEMBL194915)Show InChI InChI=1S/C17H17ClN4O2S/c18-14-4-5-15-13(17(14)21-11-8-19-9-12-21)6-10-22(15)25(23,24)16-3-1-2-7-20-16/h1-7,10,19H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475473
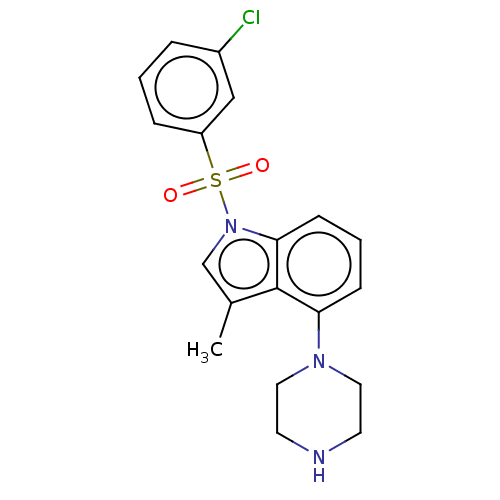
(CHEMBL194039)Show SMILES Cc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-13-23(26(24,25)16-5-2-4-15(20)12-16)18-7-3-6-17(19(14)18)22-10-8-21-9-11-22/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475479
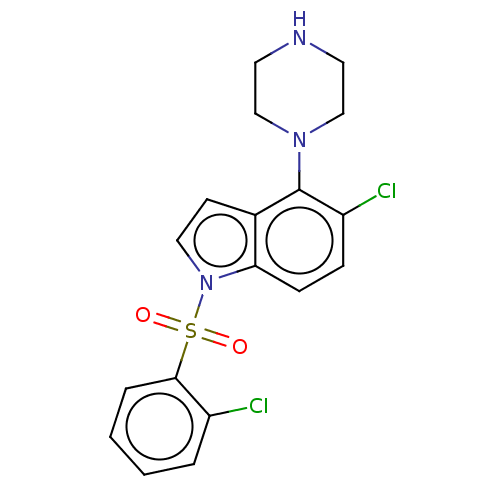
(CHEMBL371176)Show SMILES Clc1ccccc1S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-14-3-1-2-4-17(14)26(24,25)23-10-7-13-16(23)6-5-15(20)18(13)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475470

(CHEMBL370209)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,17-6-1-2-8-19-17)21-11-7-14-15(4-3-5-16(14)21)20-12-9-18-10-13-20/h1-8,11,18H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475464
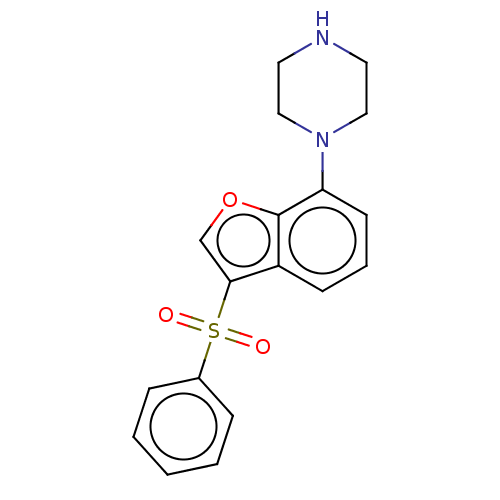
(CHEMBL197574)Show InChI InChI=1S/C18H18N2O3S/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
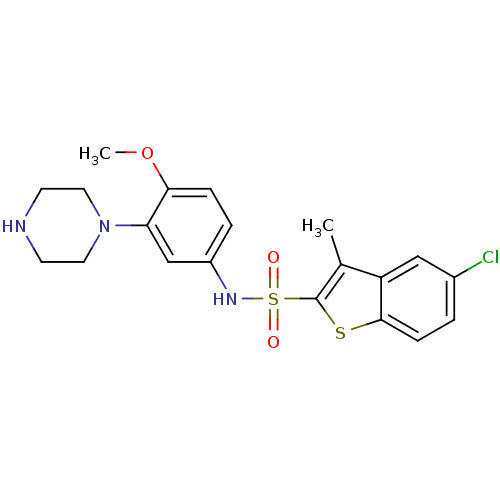
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475466
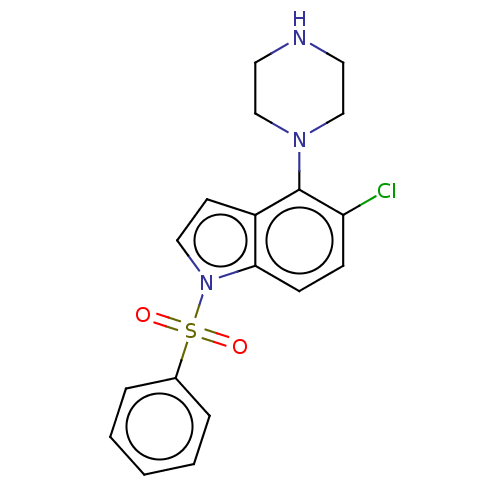
(CHEMBL193665)Show InChI InChI=1S/C18H18ClN3O2S/c19-16-6-7-17-15(18(16)21-12-9-20-10-13-21)8-11-22(17)25(23,24)14-4-2-1-3-5-14/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475471
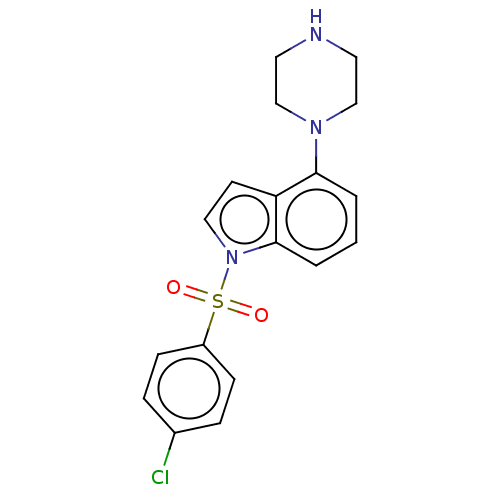
(CHEMBL371876)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-4-6-15(7-5-14)25(23,24)22-11-8-16-17(2-1-3-18(16)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475481

(CHEMBL197297)Show SMILES Cn1cc(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-22-13-18(26(24,25)15-5-2-4-14(20)12-15)16-6-3-7-17(19(16)22)23-10-8-21-9-11-23/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475478
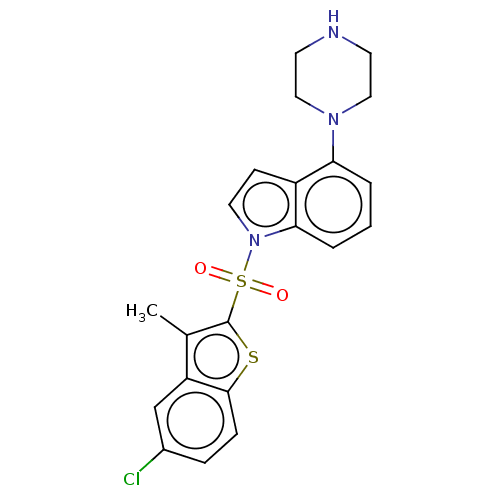
(CHEMBL196644)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2S2/c1-14-17-13-15(22)5-6-20(17)28-21(14)29(26,27)25-10-7-16-18(3-2-4-19(16)25)24-11-8-23-9-12-24/h2-7,10,13,23H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475482

(CHEMBL193379)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(ccc12)C#N Show InChI InChI=1S/C19H17ClN4O2S/c20-15-2-1-3-16(12-15)27(25,26)24-9-6-17-18(24)5-4-14(13-21)19(17)23-10-7-22-8-11-23/h1-6,9,12,22H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475472

(CHEMBL372287)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-1-3-14(4-2-13)26(24,25)23-10-7-15-17(23)6-5-16(20)18(15)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475474
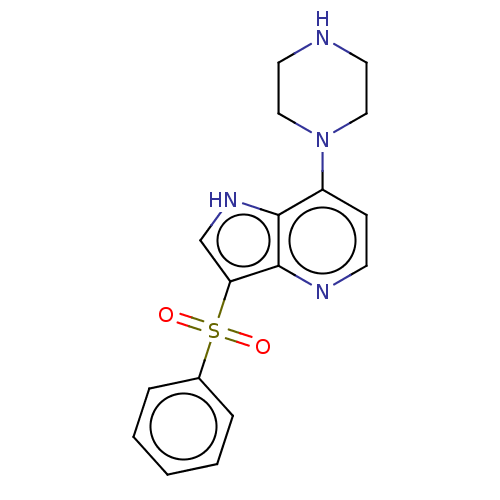
(CHEMBL426640)Show SMILES O=S(=O)(c1c[nH]c2c(ccnc12)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C17H18N4O2S/c22-24(23,13-4-2-1-3-5-13)15-12-20-16-14(6-7-19-17(15)16)21-10-8-18-9-11-21/h1-7,12,18,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 6 receptor of human caudate |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475476
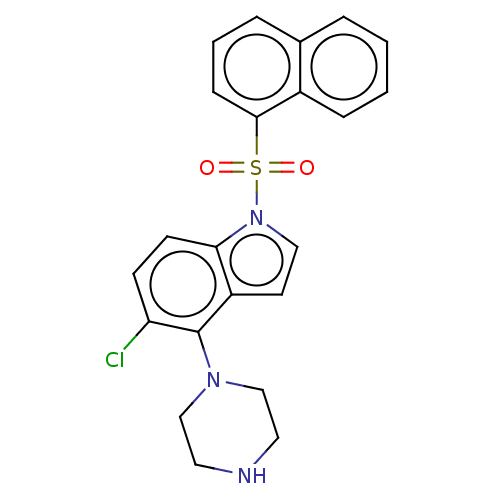
(CHEMBL196103)Show SMILES Clc1ccc2n(ccc2c1N1CCNCC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H20ClN3O2S/c23-19-8-9-20-18(22(19)25-14-11-24-12-15-25)10-13-26(20)29(27,28)21-7-3-5-16-4-1-2-6-17(16)21/h1-10,13,24H,11-12,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 6 receptor of rat striatum |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475469

(CHEMBL196524)Show SMILES Clc1ccc2n(ccc2c1N1CCNCC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H20ClN3O2S/c23-20-7-8-21-19(22(20)25-13-10-24-11-14-25)9-12-26(21)29(27,28)18-6-5-16-3-1-2-4-17(16)15-18/h1-9,12,15,24H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475468
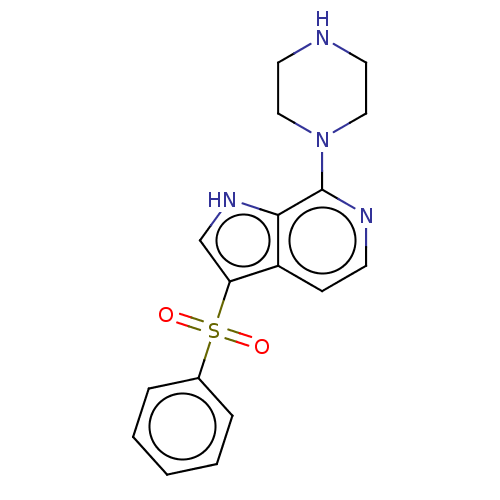
(CHEMBL366248)Show SMILES O=S(=O)(c1c[nH]c2c(nccc12)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C17H18N4O2S/c22-24(23,13-4-2-1-3-5-13)15-12-20-16-14(15)6-7-19-17(16)21-10-8-18-9-11-21/h1-7,12,18,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483581

(CHEMBL1689301)Show SMILES CC1=C(Sc2cccc(c2)C(F)(F)F)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C16H16F3NO4S/c1-9-13(12(8-24-6-5-21)15(23)20-14(9)22)25-11-4-2-3-10(7-11)16(17,18)19/h2-4,7,12,21H,5-6,8H2,1H3,(H,20,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483589
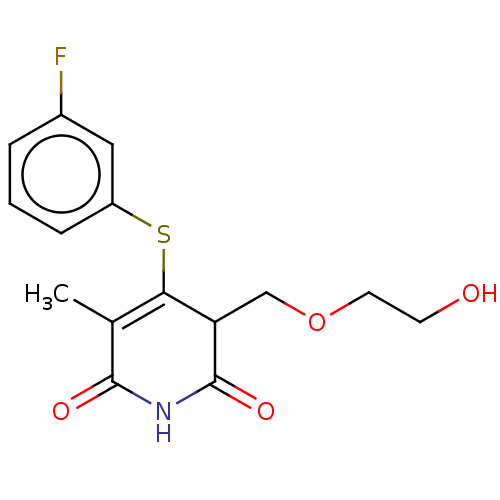
(CHEMBL1689302)Show SMILES CC1=C(Sc2cccc(F)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16FNO4S/c1-9-13(22-11-4-2-3-10(16)7-11)12(8-21-6-5-18)15(20)17-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
(Homo sapiens (Human)) | BDBM50547191
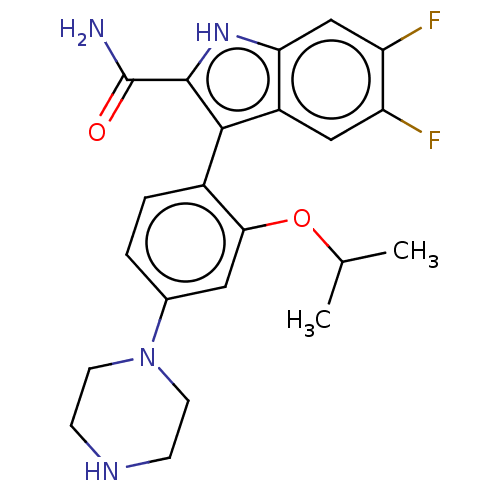
(CHEMBL4746468)Show SMILES CC(C)Oc1cc(ccc1-c1c([nH]c2cc(F)c(F)cc12)C(N)=O)N1CCNCC1 |(22.35,-9.28,;22.36,-10.82,;21.04,-11.61,;23.7,-11.58,;25.03,-10.8,;25.01,-9.26,;26.34,-8.47,;27.68,-9.23,;27.7,-10.77,;26.36,-11.55,;26.37,-13.09,;27.28,-14.35,;26.37,-15.6,;24.9,-15.12,;23.57,-15.89,;22.23,-15.12,;20.9,-15.89,;22.24,-13.58,;20.9,-12.81,;23.56,-12.81,;24.9,-13.57,;28.82,-14.35,;29.59,-13.01,;29.59,-15.68,;26.32,-6.93,;27.66,-6.14,;27.64,-4.6,;26.31,-3.84,;24.98,-4.62,;24.99,-6.17,)| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483582
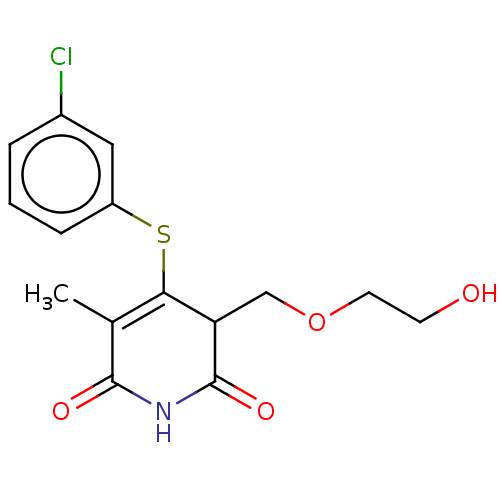
(CHEMBL1689303)Show SMILES CC1=C(Sc2cccc(Cl)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16ClNO4S/c1-9-13(22-11-4-2-3-10(16)7-11)12(8-21-6-5-18)15(20)17-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483597
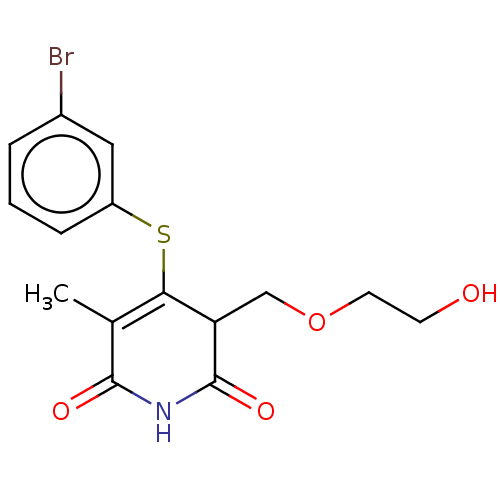
(CHEMBL1689304)Show SMILES CC1=C(Sc2cccc(Br)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16BrNO4S/c1-9-13(22-11-4-2-3-10(16)7-11)12(8-21-6-5-18)15(20)17-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
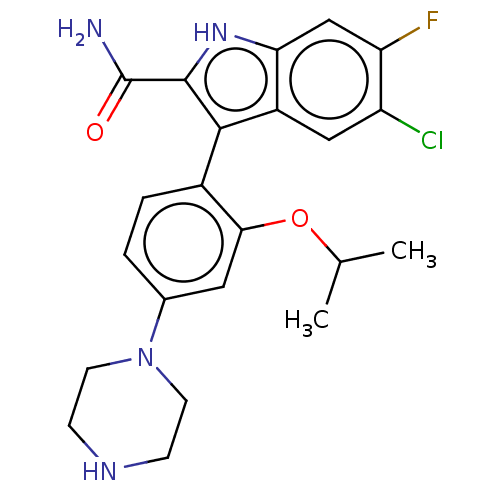
(Homo sapiens (Human)) | BDBM50547190
(CHEMBL4762949)Show SMILES CC(C)Oc1cc(ccc1-c1c([nH]c2cc(F)c(Cl)cc12)C(N)=O)N1CCNCC1 |(7.01,-9.22,;7.03,-10.76,;5.7,-11.54,;8.37,-11.52,;9.69,-10.73,;9.67,-9.19,;11,-8.4,;12.35,-9.16,;12.36,-10.7,;11.02,-11.49,;11.04,-13.03,;11.95,-14.28,;11.04,-15.54,;9.56,-15.06,;8.23,-15.83,;6.9,-15.06,;5.56,-15.83,;6.9,-13.52,;5.57,-12.75,;8.23,-12.75,;9.56,-13.51,;13.49,-14.28,;14.26,-12.95,;14.26,-15.62,;10.99,-6.86,;12.32,-6.07,;12.31,-4.54,;10.97,-3.78,;9.64,-4.56,;9.65,-6.11,)| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
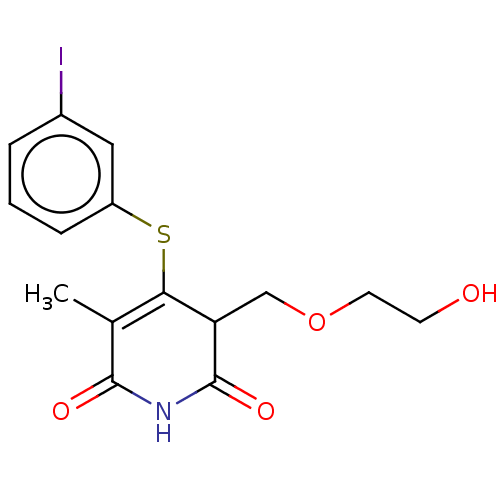
(Human immunodeficiency virus 1) | BDBM50483583
(CHEMBL1689305)Show SMILES CC1=C(Sc2cccc(I)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16INO4S/c1-9-13(22-11-4-2-3-10(16)7-11)12(8-21-6-5-18)15(20)17-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
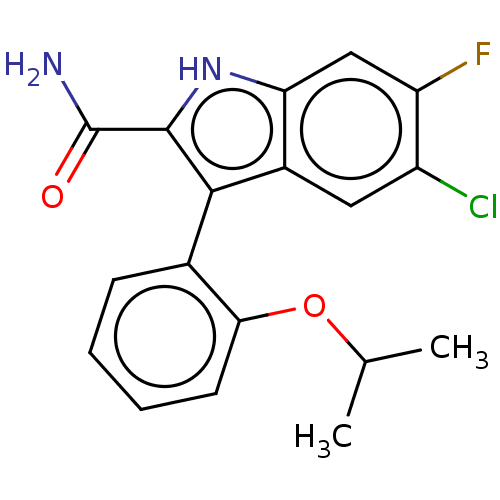
(Homo sapiens (Human)) | BDBM50547180
(CHEMBL4796852)Show SMILES CC(C)Oc1ccccc1-c1c([nH]c2cc(F)c(Cl)cc12)C(N)=O |(52.36,-5.21,;52.38,-6.75,;51.05,-7.53,;53.72,-7.51,;55.04,-6.72,;55.02,-5.18,;56.35,-4.39,;57.7,-5.15,;57.71,-6.69,;56.37,-7.48,;56.39,-9.02,;57.3,-10.27,;56.39,-11.53,;54.91,-11.05,;53.58,-11.82,;52.25,-11.05,;50.91,-11.82,;52.25,-9.51,;50.92,-8.74,;53.58,-8.74,;54.91,-9.5,;58.84,-10.27,;59.61,-8.94,;59.61,-11.61,)| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483595

(CHEMBL1689306)Show SMILES CC1=C(Sc2cccc(c2)[N+]([O-])=O)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H16N2O6S/c1-9-13(24-11-4-2-3-10(7-11)17(21)22)12(8-23-6-5-18)15(20)16-14(9)19/h2-4,7,12,18H,5-6,8H2,1H3,(H,16,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
(Homo sapiens (Human)) | BDBM50547179
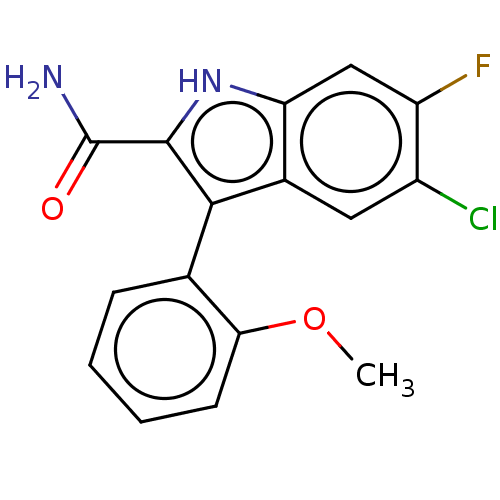
(CHEMBL4743507)Show SMILES COc1ccccc1-c1c([nH]c2cc(F)c(Cl)cc12)C(N)=O |(34.17,-6.06,;35.51,-6.82,;36.83,-6.03,;36.81,-4.5,;38.14,-3.71,;39.48,-4.46,;39.5,-6,;38.16,-6.79,;38.17,-8.33,;39.08,-9.58,;38.17,-10.84,;36.7,-10.36,;35.37,-11.13,;34.04,-10.36,;32.71,-11.13,;34.04,-8.82,;32.71,-8.05,;35.37,-8.05,;36.7,-8.81,;40.62,-9.58,;41.39,-8.25,;41.39,-10.91,)| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483584
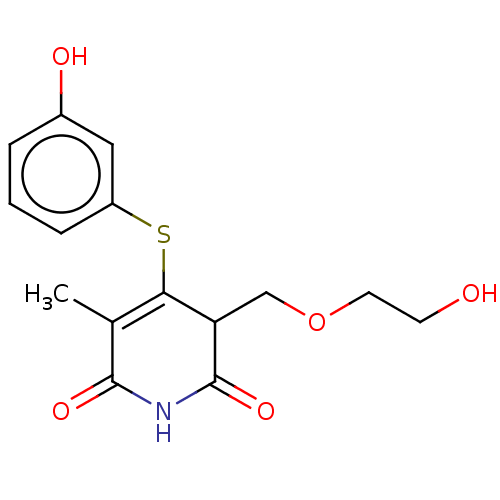
(CHEMBL1689307)Show SMILES CC1=C(Sc2cccc(O)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H17NO5S/c1-9-13(22-11-4-2-3-10(18)7-11)12(8-21-6-5-17)15(20)16-14(9)19/h2-4,7,12,17-18H,5-6,8H2,1H3,(H,16,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483593
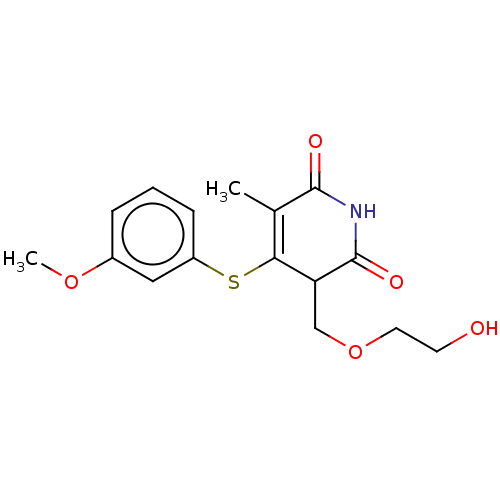
(CHEMBL1687958)Show SMILES COc1cccc(SC2=C(C)C(=O)NC(=O)C2COCCO)c1 |c:8| Show InChI InChI=1S/C16H19NO5S/c1-10-14(23-12-5-3-4-11(8-12)21-2)13(9-22-7-6-18)16(20)17-15(10)19/h3-5,8,13,18H,6-7,9H2,1-2H3,(H,17,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483585
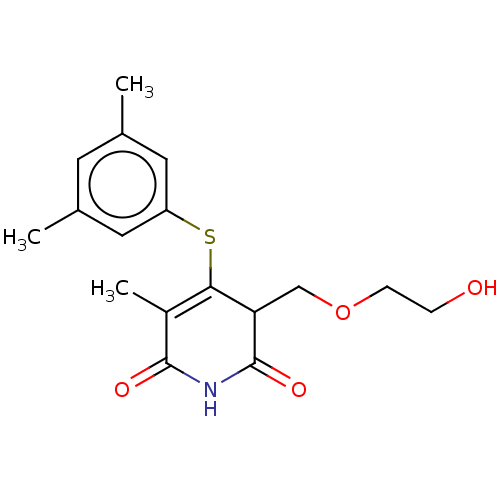
(CHEMBL1689308)Show SMILES CC1=C(Sc2cc(C)cc(C)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C17H21NO4S/c1-10-6-11(2)8-13(7-10)23-15-12(3)16(20)18-17(21)14(15)9-22-5-4-19/h6-8,14,19H,4-5,9H2,1-3H3,(H,18,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
(Homo sapiens (Human)) | BDBM50547178
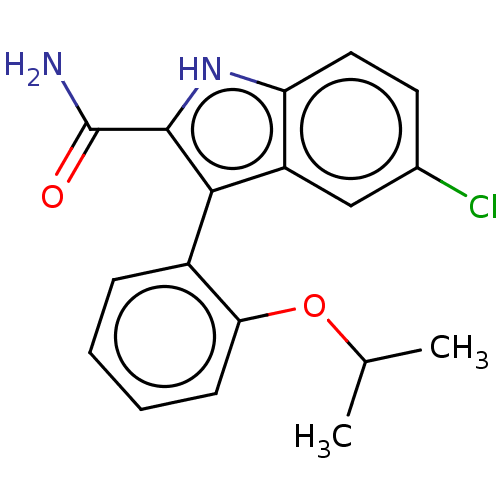
(CHEMBL4800438)Show SMILES CC(C)Oc1ccccc1-c1c([nH]c2ccc(Cl)cc12)C(N)=O |(18.66,-6.9,;19.98,-6.12,;19.97,-4.58,;21.32,-6.88,;22.65,-6.09,;22.63,-4.55,;23.96,-3.76,;25.3,-4.52,;25.32,-6.06,;23.98,-6.85,;23.99,-8.39,;24.9,-9.64,;23.99,-10.9,;22.52,-10.42,;21.19,-11.19,;19.85,-10.42,;19.86,-8.88,;18.52,-8.11,;21.18,-8.11,;22.52,-8.87,;26.44,-9.64,;27.21,-8.31,;27.21,-10.98,)| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
(Homo sapiens (Human)) | BDBM50547187
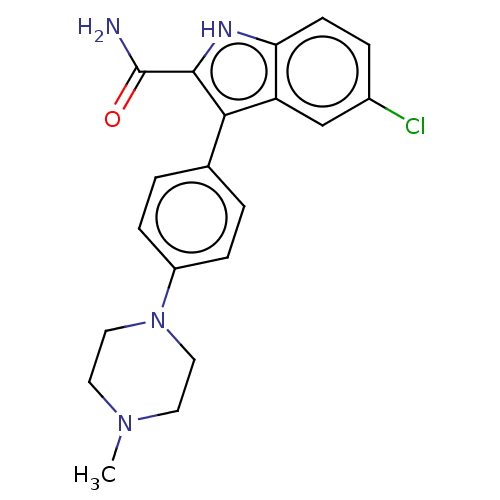
(CHEMBL4791887)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1c([nH]c2ccc(Cl)cc12)C(N)=O | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483586
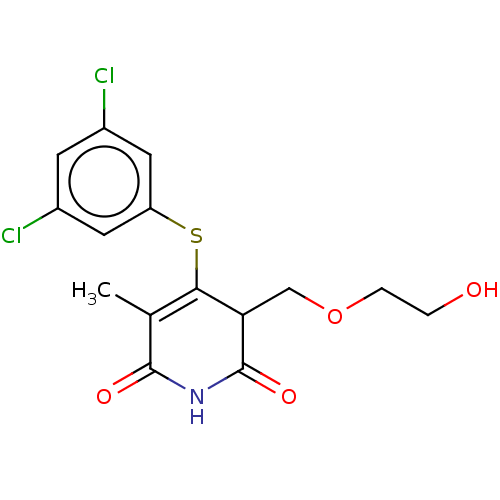
(CHEMBL1689309)Show SMILES CC1=C(Sc2cc(Cl)cc(Cl)c2)C(COCCO)C(=O)NC1=O |c:1| Show InChI InChI=1S/C15H15Cl2NO4S/c1-8-13(23-11-5-9(16)4-10(17)6-11)12(7-22-3-2-19)15(21)18-14(8)20/h4-6,12,19H,2-3,7H2,1H3,(H,18,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483574

(CHEMBL1689310)Show SMILES CC(C)C1=C(Sc2ccccc2)C(COCCO)C(=O)NC1=O |c:3| Show InChI InChI=1S/C17H21NO4S/c1-11(2)14-15(23-12-6-4-3-5-7-12)13(10-22-9-8-19)16(20)18-17(14)21/h3-7,11,13,19H,8-10H2,1-2H3,(H,18,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Mus musculus (Mouse)) | BDBM50547191

(CHEMBL4746468)Show SMILES CC(C)Oc1cc(ccc1-c1c([nH]c2cc(F)c(F)cc12)C(N)=O)N1CCNCC1 |(22.35,-9.28,;22.36,-10.82,;21.04,-11.61,;23.7,-11.58,;25.03,-10.8,;25.01,-9.26,;26.34,-8.47,;27.68,-9.23,;27.7,-10.77,;26.36,-11.55,;26.37,-13.09,;27.28,-14.35,;26.37,-15.6,;24.9,-15.12,;23.57,-15.89,;22.23,-15.12,;20.9,-15.89,;22.24,-13.58,;20.9,-12.81,;23.56,-12.81,;24.9,-13.57,;28.82,-14.35,;29.59,-13.01,;29.59,-15.68,;26.32,-6.93,;27.66,-6.14,;27.64,-4.6,;26.31,-3.84,;24.98,-4.62,;24.99,-6.17,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 in mouse B cells assessed as reduction in IgG secretion |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Microtubule-associated protein 2
(Homo sapiens (Human)) | BDBM50547186

(CHEMBL4756420)Show SMILES NC(=O)c1[nH]c2ccc(Cl)cc2c1-c1ccc(cc1)N1CCOCC1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483587

(CHEMBL1689312)Show SMILES CC(C)C1=C(Sc2cc(C)cc(C)c2)C(COCCO)C(=O)NC1=O |c:3| Show InChI InChI=1S/C19H25NO4S/c1-11(2)16-17(25-14-8-12(3)7-13(4)9-14)15(10-24-6-5-21)18(22)20-19(16)23/h7-9,11,15,21H,5-6,10H2,1-4H3,(H,20,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
(Homo sapiens (Human)) | BDBM50547173
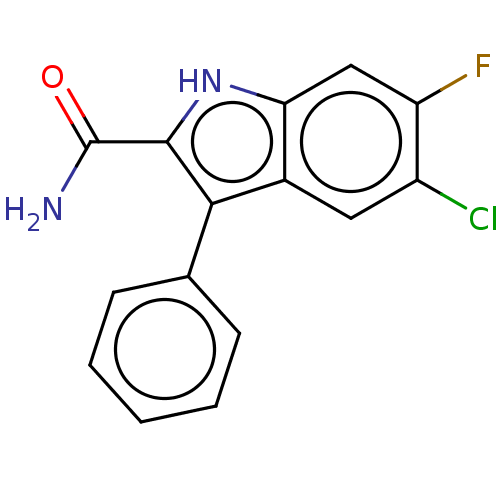
(CHEMBL4751717) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483590

(CHEMBL1689311)Show SMILES CCC1=C(Sc2cc(C)cc(C)c2)C(COCCO)C(=O)NC1=O |c:2| Show InChI InChI=1S/C18H23NO4S/c1-4-14-16(24-13-8-11(2)7-12(3)9-13)15(10-23-6-5-20)18(22)19-17(14)21/h7-9,15,20H,4-6,10H2,1-3H3,(H,19,21,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GVK Biosciences Private Limited
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 reverse transcriptase |
Eur J Med Chem 46: 851-9 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.022
BindingDB Entry DOI: 10.7270/Q2HD7ZGX |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
(Homo sapiens (Human)) | BDBM50547175
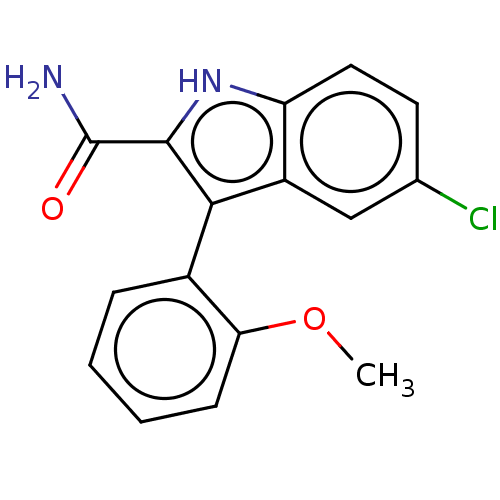
(CHEMBL4777059)Show SMILES COc1ccccc1-c1c([nH]c2ccc(Cl)cc12)C(N)=O |(50.41,-49.94,;51.75,-50.69,;53.08,-49.91,;53.06,-48.37,;54.39,-47.58,;55.73,-48.34,;55.75,-49.88,;54.41,-50.66,;54.42,-52.2,;55.33,-53.46,;54.42,-54.71,;52.95,-54.23,;51.62,-55,;50.29,-54.23,;50.29,-52.69,;48.96,-51.92,;51.62,-51.92,;52.95,-52.68,;56.87,-53.46,;57.64,-52.13,;57.64,-54.79,)| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MetAP2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127533
BindingDB Entry DOI: 10.7270/Q2N58QZH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data