

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrin alpha-L (Mus musculus) | BDBM50426077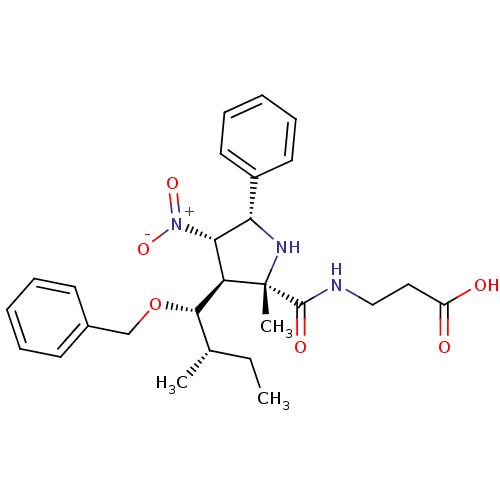 (CHEMBL2315999) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ikerchem Ltd. Curated by ChEMBL | Assay Description Inhibition of LFA-1/ICAM-1 interaction in mouse Lim51b cells assessed as PMA-induced adhesion to ICAM-1 expressing HSE cells measured per 10'5 cells ... | J Med Chem 56: 735-47 (2013) Article DOI: 10.1021/jm3016848 BindingDB Entry DOI: 10.7270/Q2862HSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50426078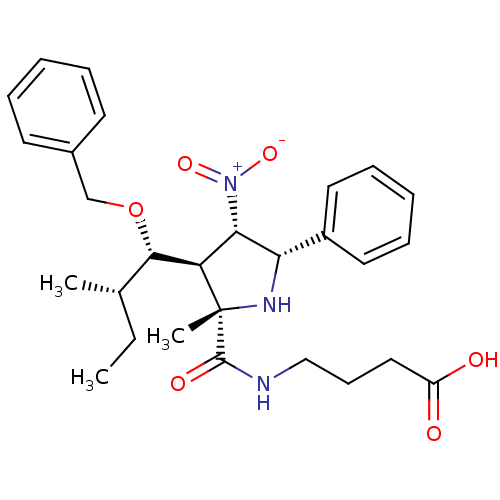 (CHEMBL2316248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ikerchem Ltd. Curated by ChEMBL | Assay Description Binding affinity to I-domain of human integrin alphaL (amino acid residues 128 to 307) plus initial methionine expressed in Escherichia coli by NMR s... | J Med Chem 56: 735-47 (2013) Article DOI: 10.1021/jm3016848 BindingDB Entry DOI: 10.7270/Q2862HSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50236238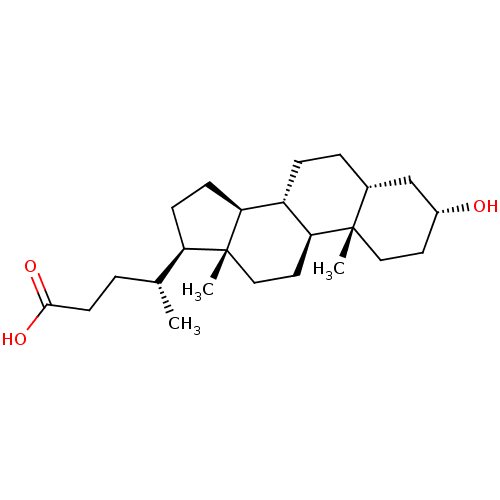 ((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity to N-terminal His-tagged human VDR LBD canonical site (118 to 427) by direct isothermal titration calorimetric analysis | J Med Chem 57: 4710-9 (2014) Article DOI: 10.1021/jm5002524 BindingDB Entry DOI: 10.7270/Q2PV6MX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50236238 ((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 9.52E+3 | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity to N-terminal His-tagged human VDR LBD low-affinity site (118 to 427) by direct isothermal titration calorimetric analysis | J Med Chem 57: 4710-9 (2014) Article DOI: 10.1021/jm5002524 BindingDB Entry DOI: 10.7270/Q2PV6MX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50236238 ((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity to N-terminal His-tagged human VDR LBD (118 to 427) by reverse isothermal titration calorimetric analysis | J Med Chem 57: 4710-9 (2014) Article DOI: 10.1021/jm5002524 BindingDB Entry DOI: 10.7270/Q2PV6MX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397073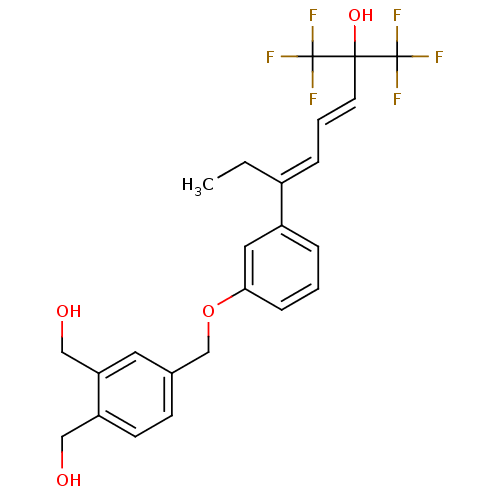 (CHEMBL2171448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Transactivation of GAL4-fused VDR ligand binding domain expressed in human HeLa cells after 16 hrs by luciferase reporter gene assay | J Med Chem 55: 8440-9 (2012) Article DOI: 10.1021/jm300858s BindingDB Entry DOI: 10.7270/Q2X63P3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Transactivation of full length human VDR expressed in human HeLa cells assessed as increase in CYP24 transcription after 18 hrs by luciferase reporte... | J Med Chem 55: 8440-9 (2012) Article DOI: 10.1021/jm300858s BindingDB Entry DOI: 10.7270/Q2X63P3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397075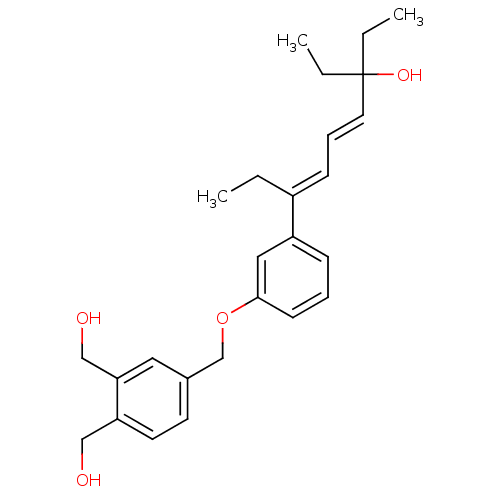 (CHEMBL2171446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Transactivation of full length human VDR expressed in human HeLa cells assessed as increase in CYP24 transcription after 18 hrs by luciferase reporte... | J Med Chem 55: 8440-9 (2012) Article DOI: 10.1021/jm300858s BindingDB Entry DOI: 10.7270/Q2X63P3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397074 (CHEMBL2171447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Transactivation of full length human VDR expressed in human HeLa cells assessed as increase in CYP24 transcription after 18 hrs by luciferase reporte... | J Med Chem 55: 8440-9 (2012) Article DOI: 10.1021/jm300858s BindingDB Entry DOI: 10.7270/Q2X63P3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397076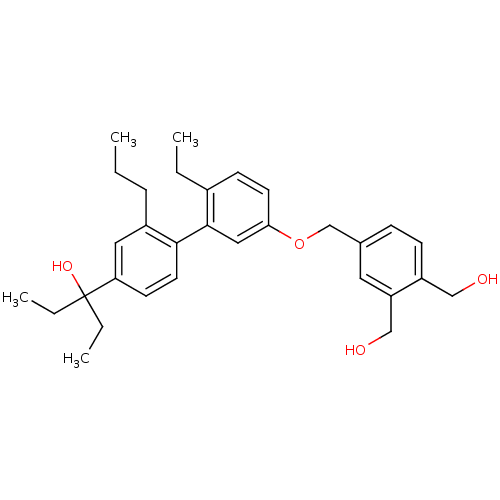 (CHEMBL2171449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Transactivation of full length human VDR expressed in human HeLa cells assessed as increase in CYP24 transcription after 18 hrs by luciferase reporte... | J Med Chem 55: 8440-9 (2012) Article DOI: 10.1021/jm300858s BindingDB Entry DOI: 10.7270/Q2X63P3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397077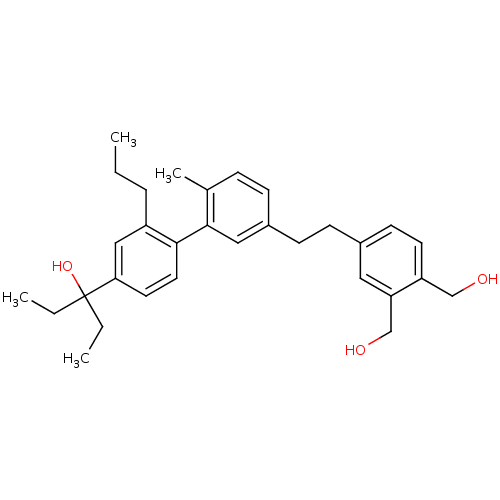 (CHEMBL2171450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Transactivation of full length human VDR expressed in human HeLa cells assessed as increase in CYP24 transcription after 18 hrs by luciferase reporte... | J Med Chem 55: 8440-9 (2012) Article DOI: 10.1021/jm300858s BindingDB Entry DOI: 10.7270/Q2X63P3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM34168 (LOVASTATIN | MLS000069585 | SMR000058779 | US91151...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a |
Ikerchem Ltd. Curated by ChEMBL | Assay Description Binding affinity to I-domain of human integrin alphaL (amino acid residues 128 to 307) plus initial methionine expressed in Escherichia coli by NMR s... | J Med Chem 56: 735-47 (2013) Article DOI: 10.1021/jm3016848 BindingDB Entry DOI: 10.7270/Q2862HSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50426077 (CHEMBL2315999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a |
Ikerchem Ltd. Curated by ChEMBL | Assay Description Binding affinity to I-domain of human integrin alphaL (amino acid residues 128 to 307) plus initial methionine expressed in Escherichia coli by NMR s... | J Med Chem 56: 735-47 (2013) Article DOI: 10.1021/jm3016848 BindingDB Entry DOI: 10.7270/Q2862HSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397074 (CHEMBL2171447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Transactivation of GAL4-fused VDR ligand binding domain expressed in human HeLa cells after 16 hrs by luciferase reporter gene assay | J Med Chem 55: 8440-9 (2012) Article DOI: 10.1021/jm300858s BindingDB Entry DOI: 10.7270/Q2X63P3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Transactivation of GAL4-fused VDR ligand binding domain expressed in human HeLa cells after 16 hrs by luciferase reporter gene assay | J Med Chem 55: 8440-9 (2012) Article DOI: 10.1021/jm300858s BindingDB Entry DOI: 10.7270/Q2X63P3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||