

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320127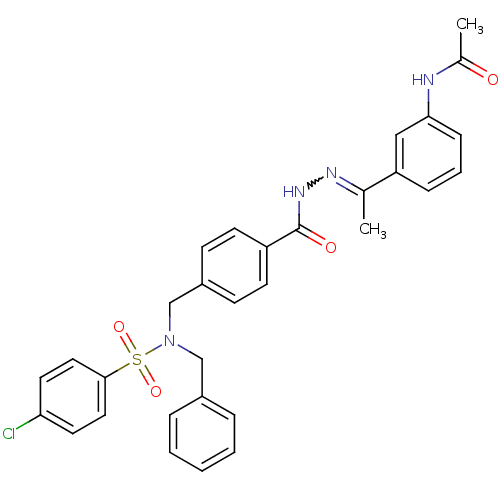 (CHEMBL1085565 | N-(3-(1-(2-(4-((N-benzyl-4-chlorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Displacement of labeled ITAC from CXCR3 | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320127 (CHEMBL1085565 | N-(3-(1-(2-(4-((N-benzyl-4-chlorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 348 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Displacement of labeled IP10 from CXCR3 | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320145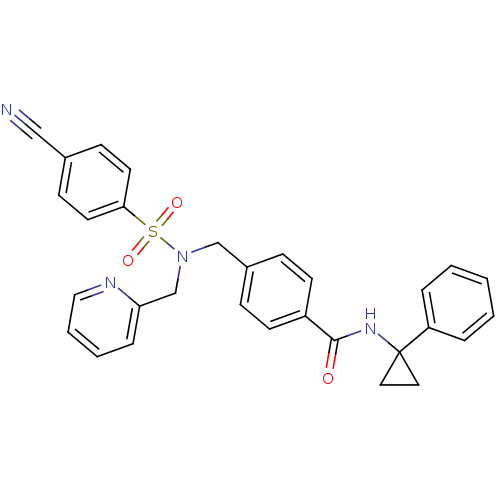 (4-((4-cyano-N-(pyridin-2-ylmethyl)phenylsulfonamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320144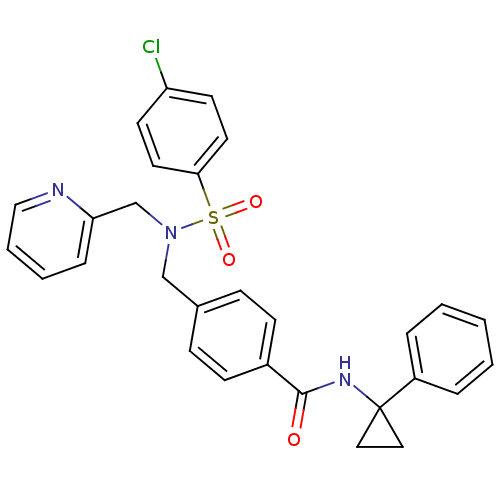 (4-((4-chloro-N-(pyridin-2-ylmethyl)phenylsulfonami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320139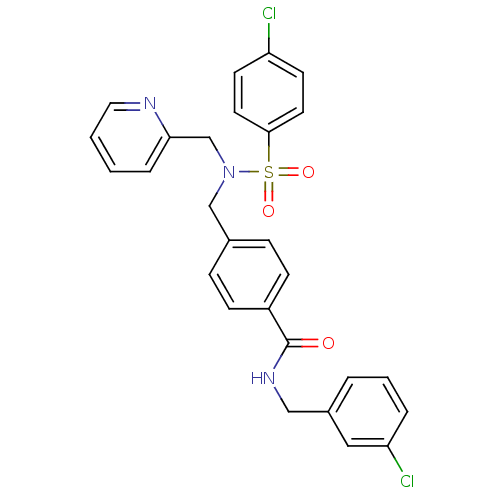 (4-((4-chloro-N-(pyridin-2-ylmethyl)phenylsulfonami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320166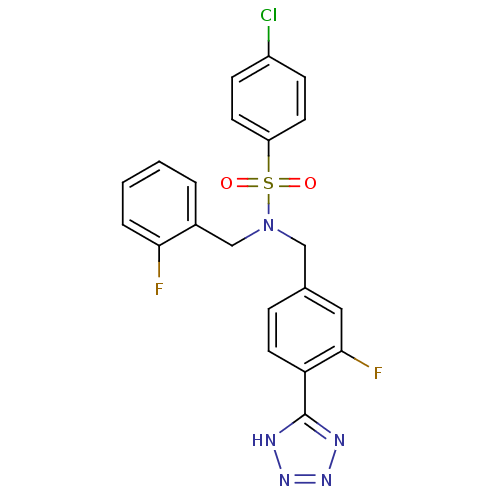 (4-chloro-N-(3-fluoro-4-(1H-tetrazol-5-yl)benzyl)-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320164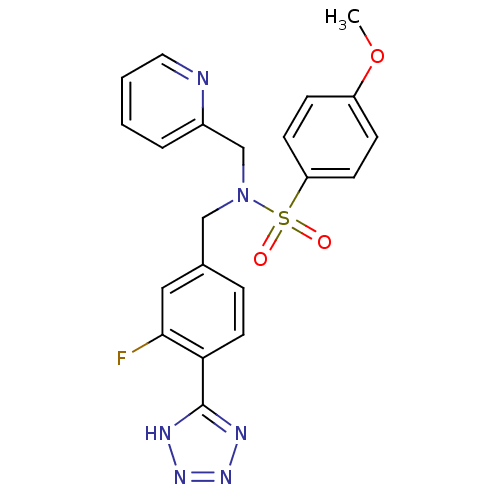 (CHEMBL1085508 | N-(3-fluoro-4-(1H-tetrazol-5-yl)be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320168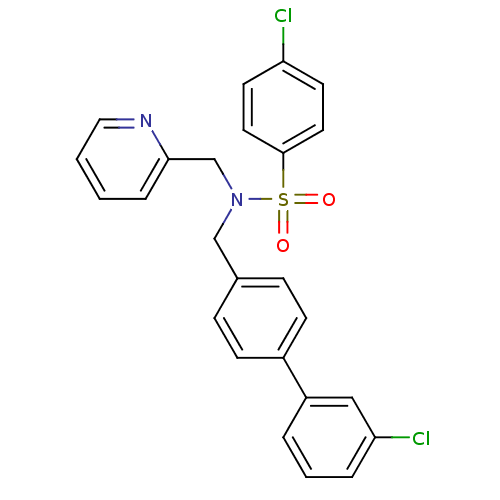 (4-chloro-N-((3'-chlorobiphenyl-4-yl)methyl)-N-(pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320165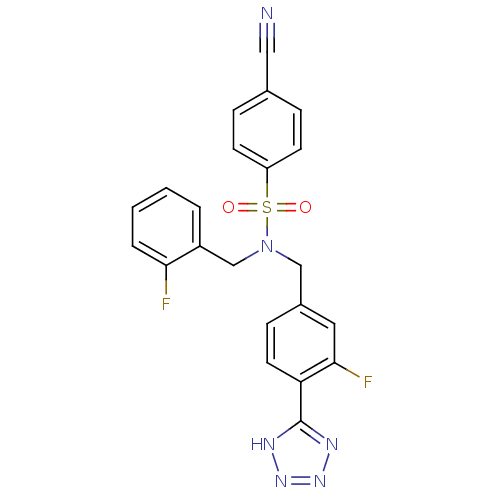 (4-cyano-N-(3-fluoro-4-(1H-tetrazol-5-yl)benzyl)-N-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320131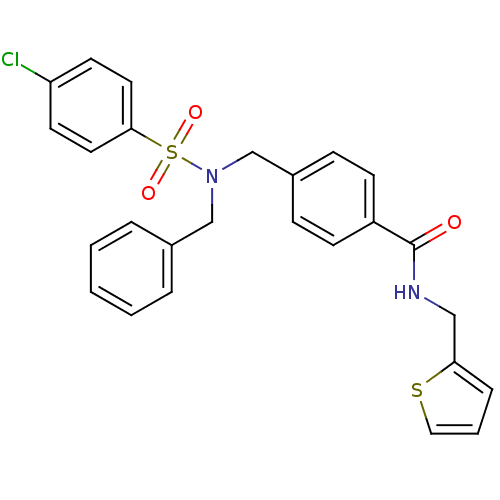 (4-((N-benzyl-4-chlorophenylsulfonamido)methyl)-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320138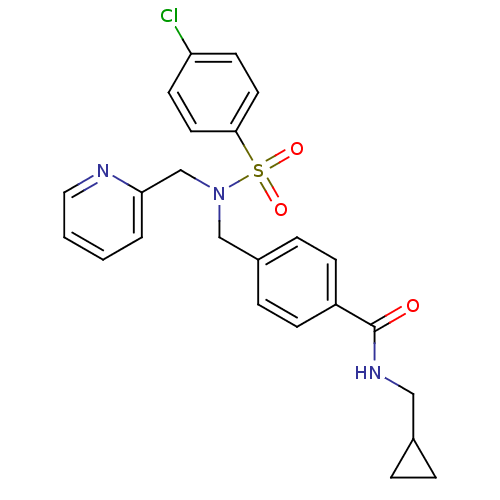 (4-((4-chloro-N-(pyridin-2-ylmethyl)phenylsulfonami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320171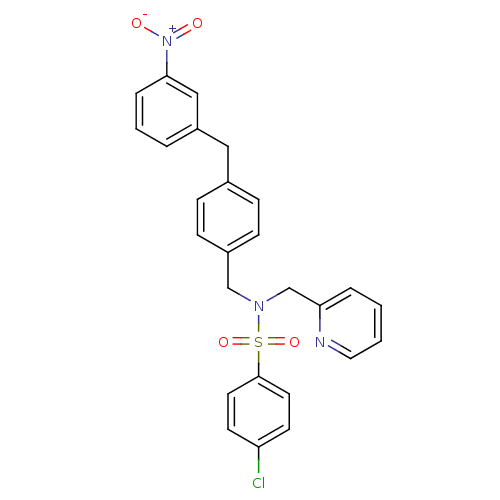 (4-chloro-N-(4-(3-nitrobenzyl)benzyl)-N-(pyridin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320170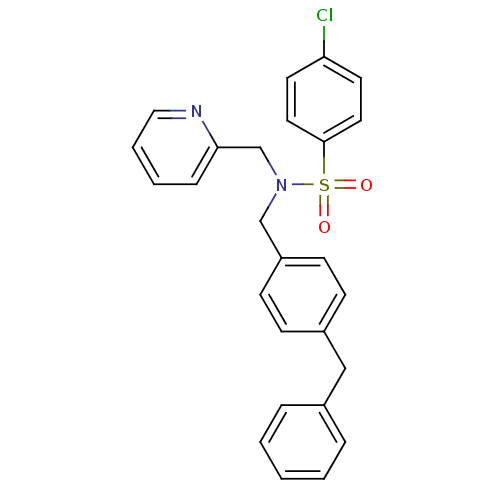 (CHEMBL1086280 | N-(4-benzylbenzyl)-4-chloro-N-(pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320156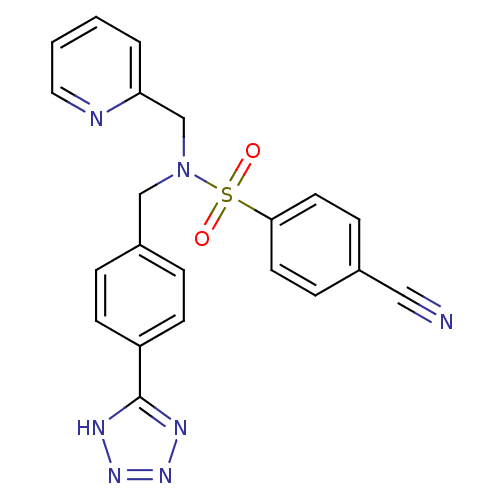 (CHEMBL1083913 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320167 (CHEMBL1082361 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 236 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320132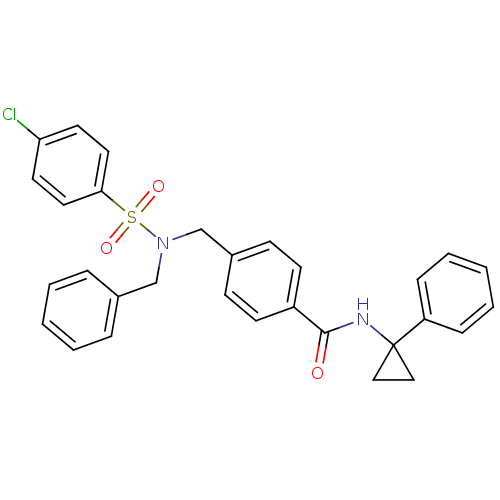 (4-((N-benzyl-4-chlorophenylsulfonamido)methyl)-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320162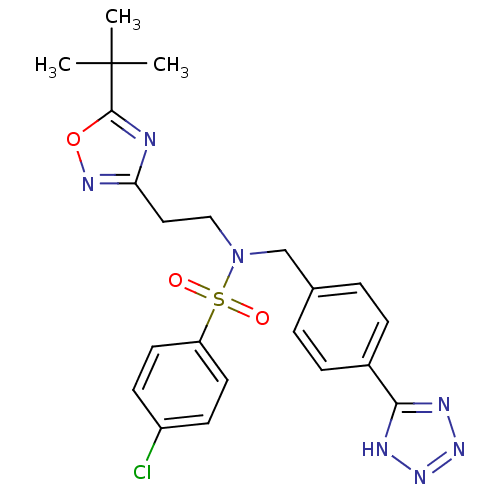 (CHEMBL1083310 | N-(4-(1H-tetrazol-5-yl)benzyl)-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320169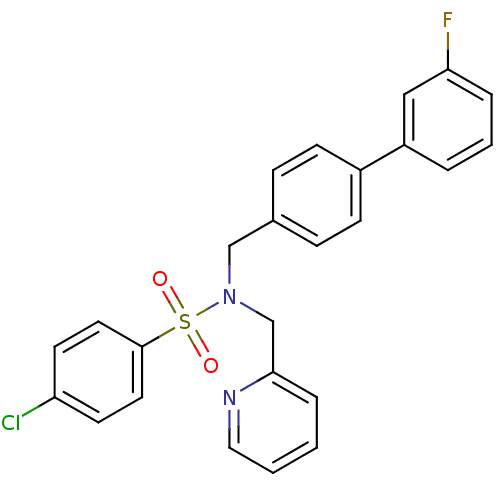 (4-chloro-N-((3'-fluorobiphenyl-4-yl)methyl)-N-(pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320135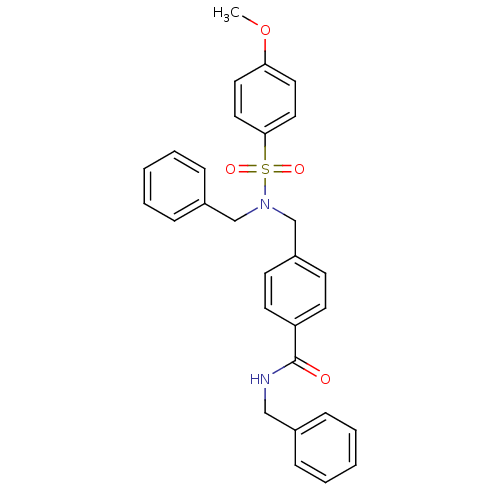 (CHEMBL1085258 | N-benzyl-4-((N-benzyl-4-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320141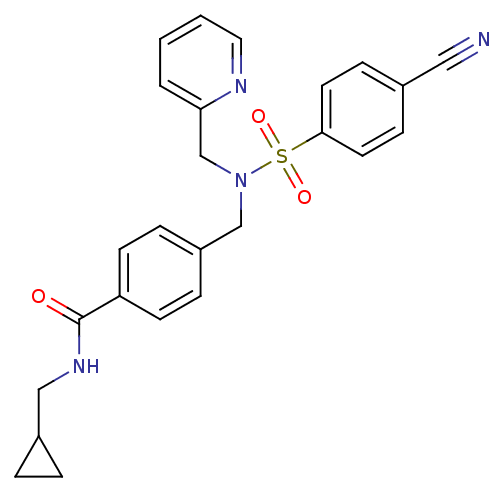 (4-((4-cyano-N-(pyridin-2-ylmethyl)phenylsulfonamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320163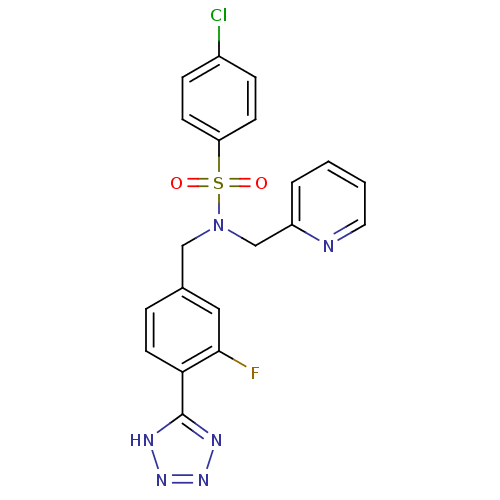 (4-chloro-N-(3-fluoro-4-(1H-tetrazol-5-yl)benzyl)-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320153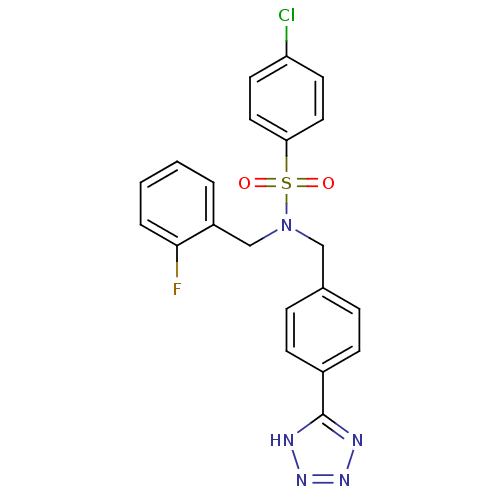 (CHEMBL1082648 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320152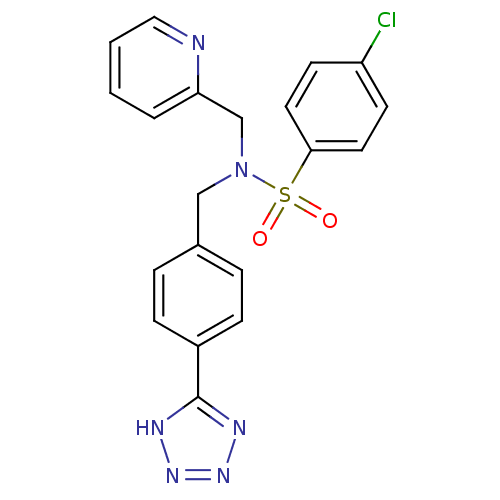 (CHEMBL1082641 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320142 (4-((4-chloro-N-(2-fluorobenzyl)phenylsulfonamido)m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320149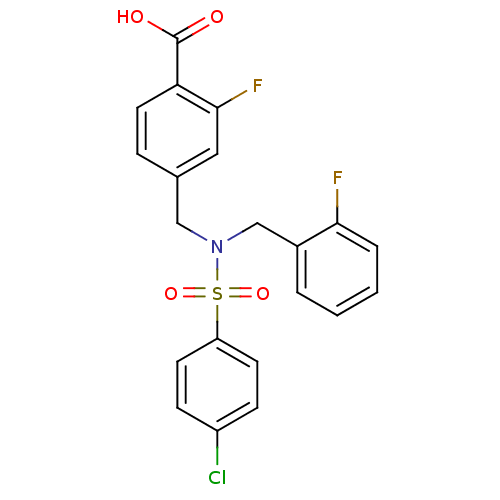 (4-((4-chloro-N-(2-fluorobenzyl)phenylsulfonamido)m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320127 (CHEMBL1085565 | N-(3-(1-(2-(4-((N-benzyl-4-chlorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 538 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320157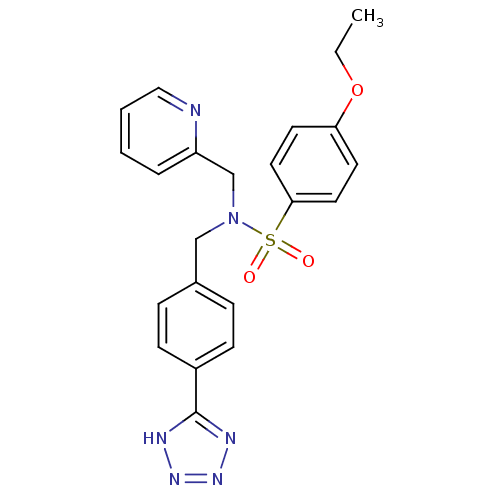 (CHEMBL1084198 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320136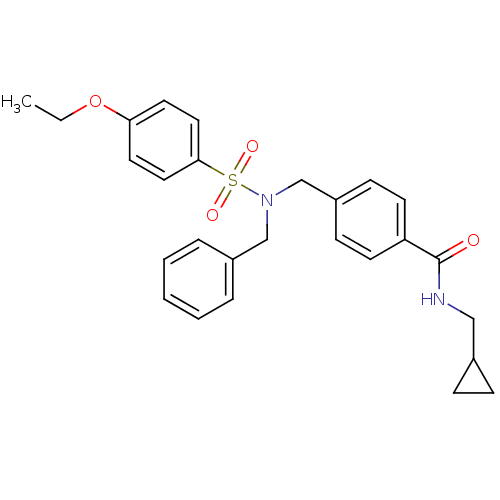 (4-((N-benzyl-4-ethoxyphenylsulfonamido)methyl)-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 673 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320151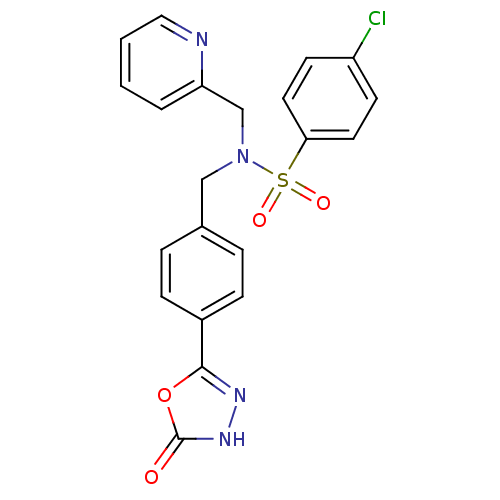 (4-chloro-N-(4-(5-hydroxy-1,3,4-oxadiazol-2-yl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 695 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320134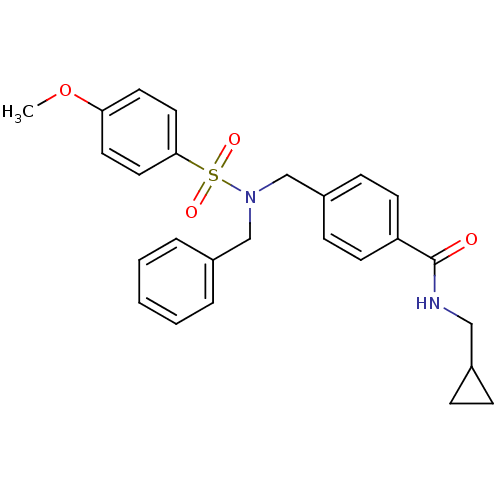 (4-((N-benzyl-4-methoxyphenylsulfonamido)methyl)-N-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 697 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320155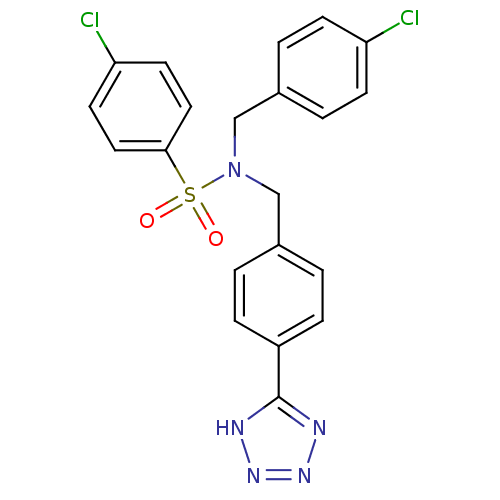 (CHEMBL1086590 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 882 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320158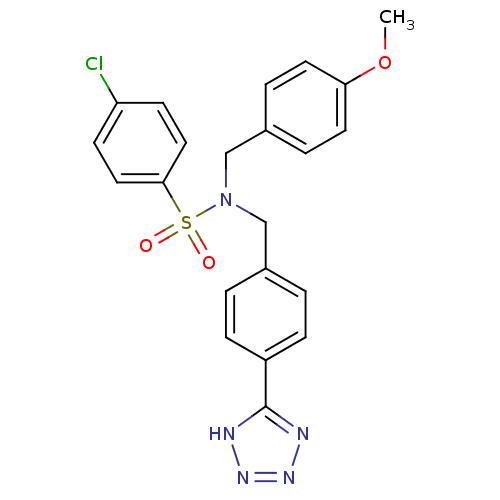 (CHEMBL1084199 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 885 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320161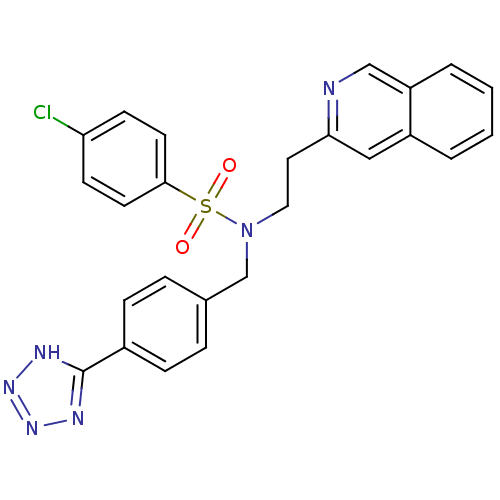 (CHEMBL1083309 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 911 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320143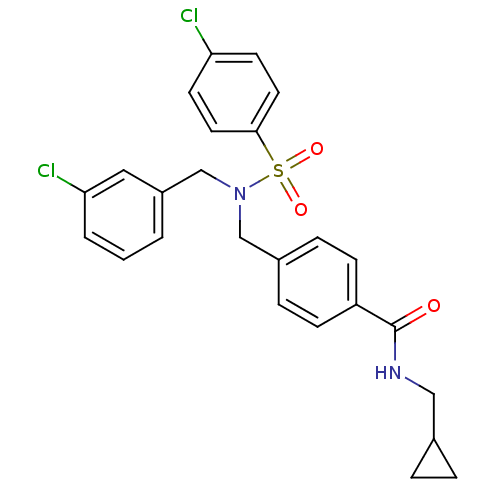 (4-((4-chloro-N-(3-chlorobenzyl)phenylsulfonamido)m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320150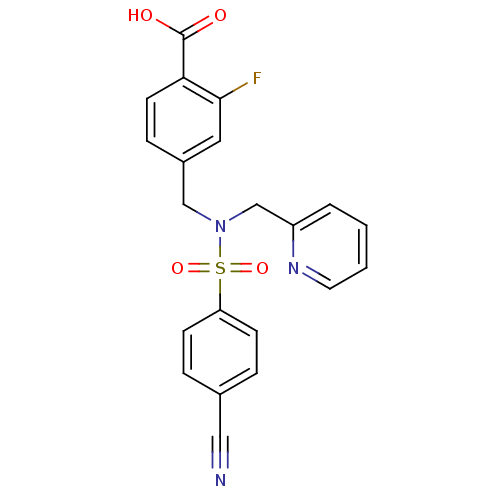 (4-((4-cyano-N-(pyridin-2-ylmethyl)phenylsulfonamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320148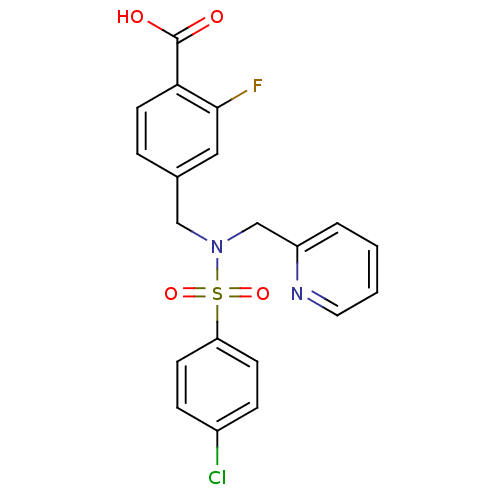 (4-((4-chloro-N-(pyridin-2-ylmethyl)phenylsulfonami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320140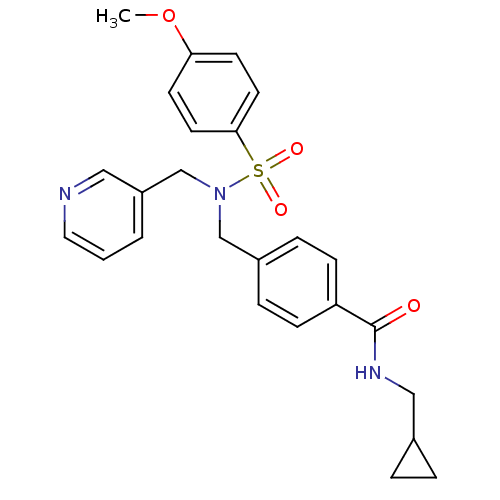 (CHEMBL1085998 | N-(cyclopropylmethyl)-4-((4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320154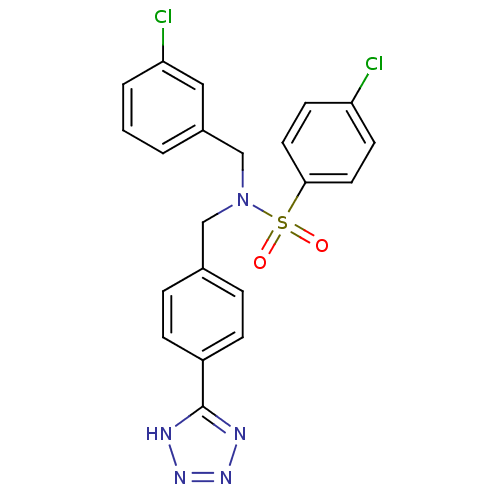 (CHEMBL1082649 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320130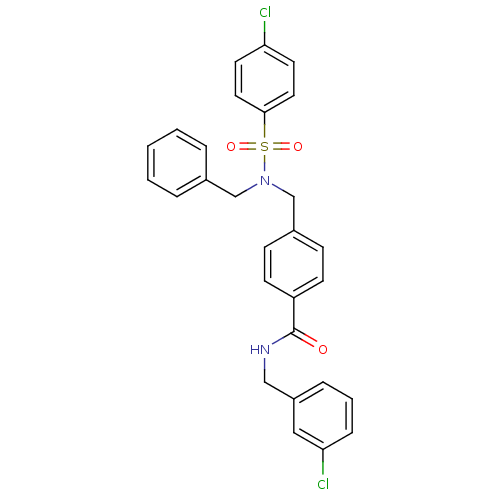 (4-((N-benzyl-4-chlorophenylsulfonamido)methyl)-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320159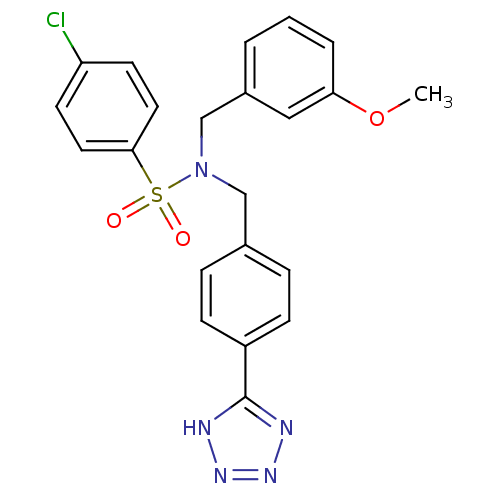 (CHEMBL1082660 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320129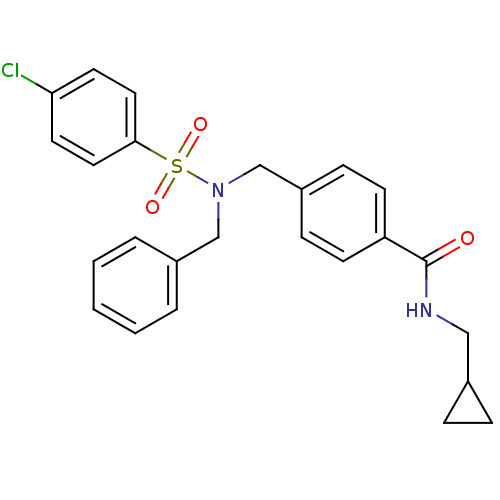 (4-((N-benzyl-4-chlorophenylsulfonamido)methyl)-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320128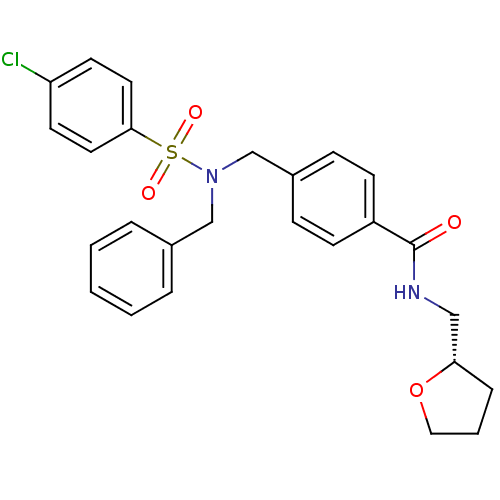 ((S)-4-((N-benzyl-4-chlorophenylsulfonamido)methyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320133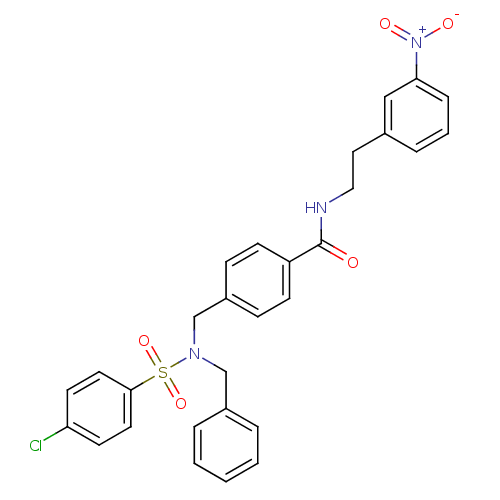 (4-((N-benzyl-4-chlorophenylsulfonamido)methyl)-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320160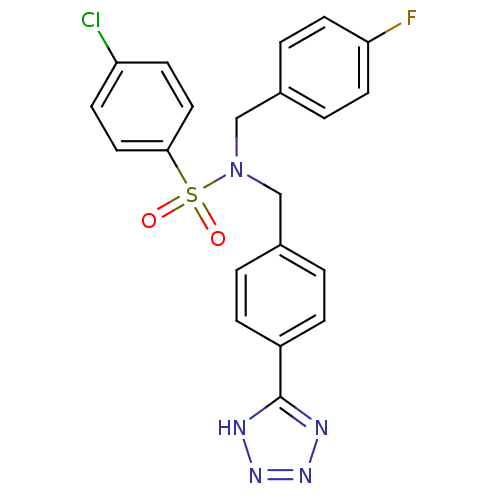 (CHEMBL1082995 | N-(4-(1H-tetrazol-5-yl)benzyl)-4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320146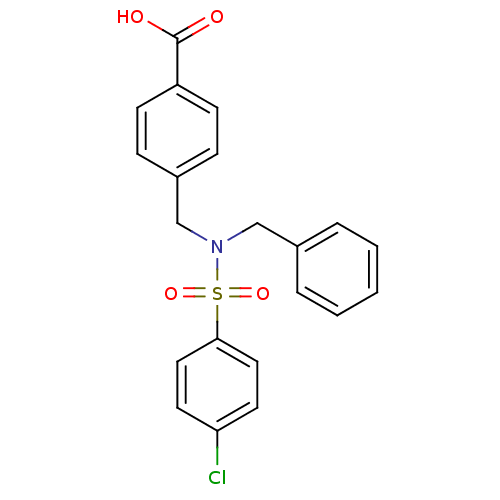 (4-((N-benzyl-4-chlorophenylsulfonamido)methyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320137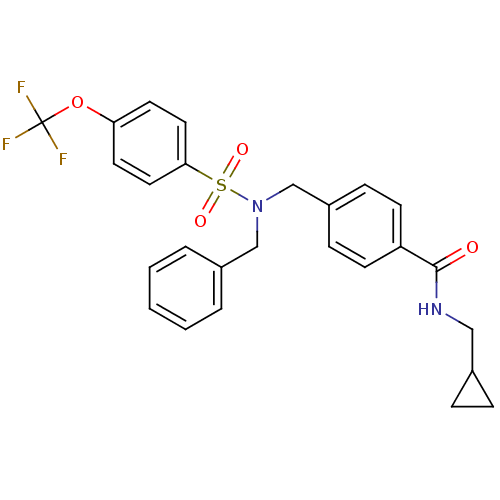 (4-((N-benzyl-4-(trifluoromethoxy)phenylsulfonamido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50320147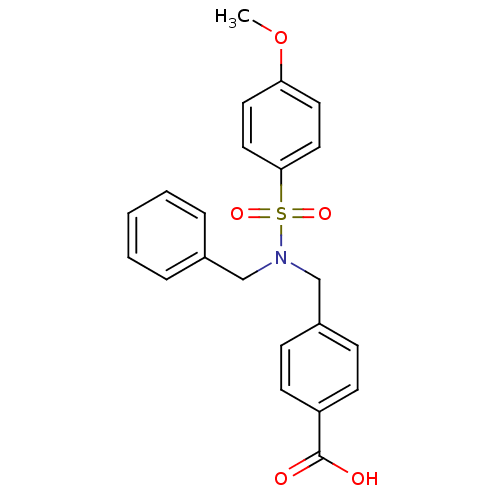 (4-((N-benzyl-4-methoxyphenylsulfonamido)methyl)ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of IP10-induced chemotaxis after 4 hrs | Bioorg Med Chem Lett 20: 3614-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.113 BindingDB Entry DOI: 10.7270/Q2668DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||