

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50093602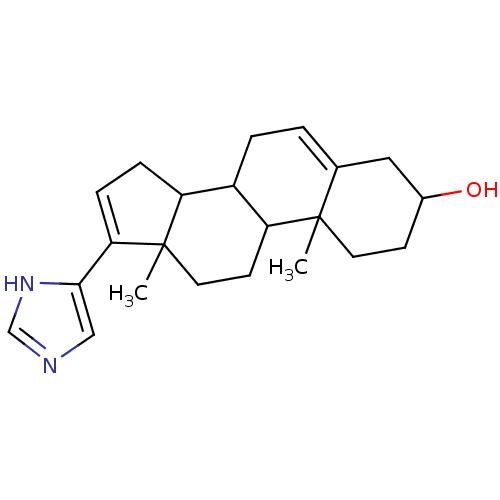 (17-(1H-Imidazol-4-yl)-10,13-dimethyl-2,3,4,7,8,9,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of human Cytochrome P450 17 | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedione | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cells | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409034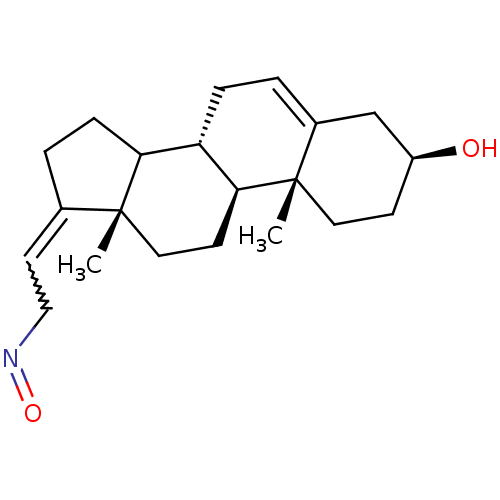 (CHEMBL2112731) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50093600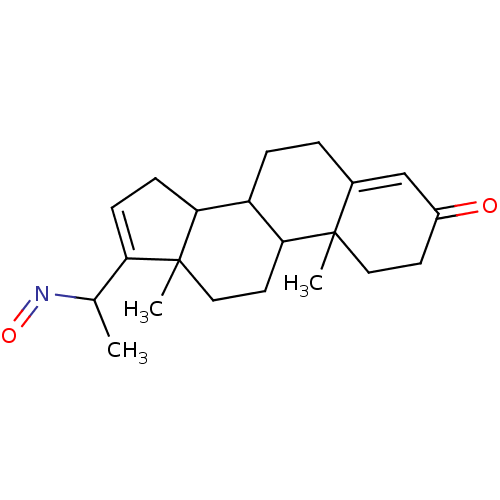 (17-(1-Hydroxyimino-ethyl)-10,13-dimethyl-1,2,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8935 (5-[(6-methoxy-1-methyl-3,4-dihydronaphthalen-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8951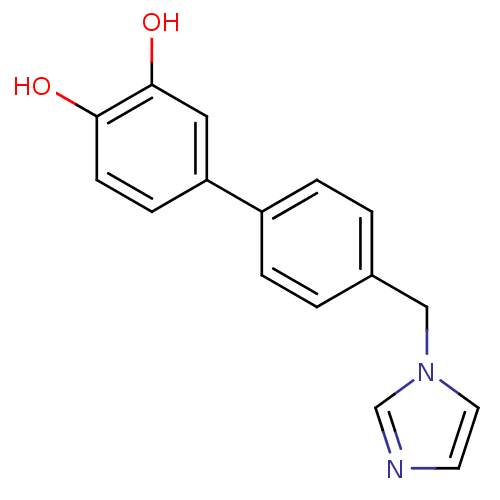 (4-Imidazol-1-ylmethyl-biphenyl-3,4-diol | 4-[4-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50409033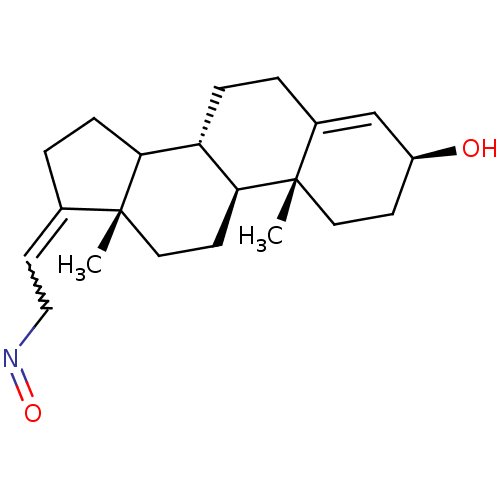 (CHEMBL2112735) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409032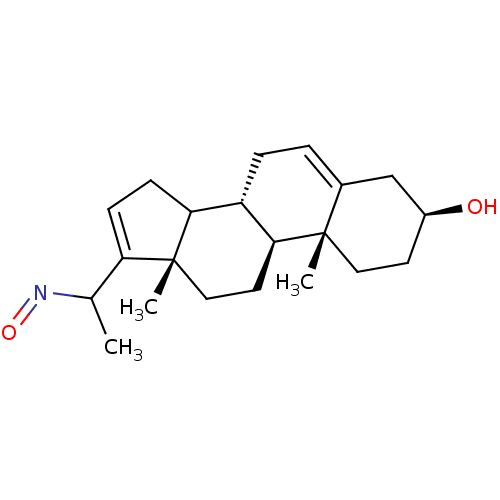 (CHEMBL2112737) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409033 (CHEMBL2112735) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50093602 (17-(1H-Imidazol-4-yl)-10,13-dimethyl-2,3,4,7,8,9,1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of rat Cytochrome P450 17 | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50093591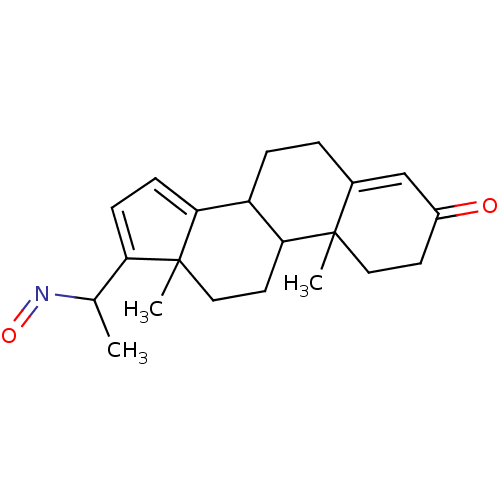 (17-(1-Hydroxyimino-ethyl)-10,13-dimethyl-1,2,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409030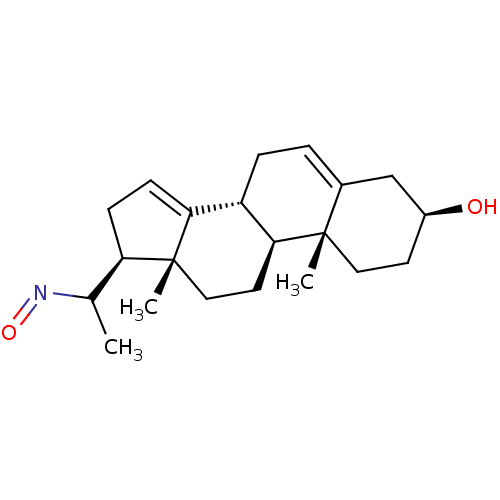 (CHEMBL2112747) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409034 (CHEMBL2112731) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50093603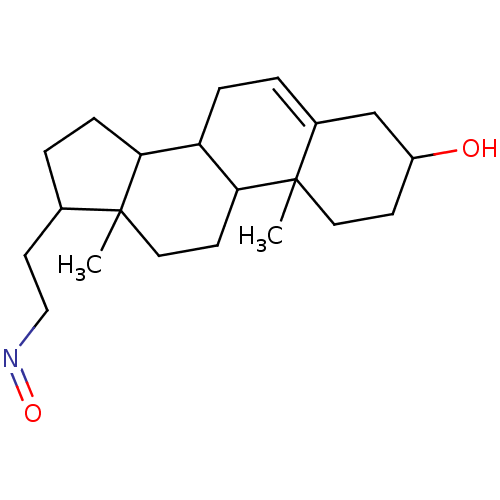 ((3-Hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50093590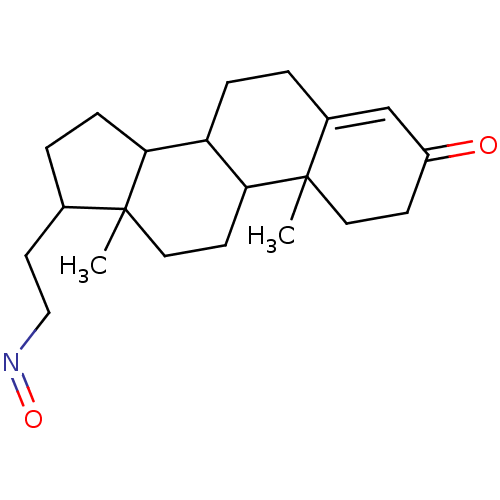 ((10,13-Dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50093590 ((10,13-Dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409030 (CHEMBL2112747) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50093598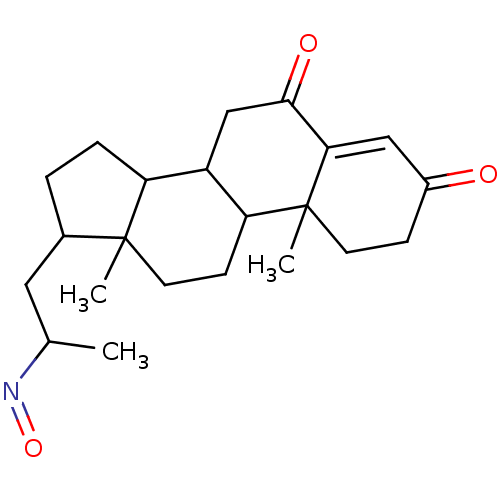 (17-(2-Hydroxyimino-propyl)-10,13-dimethyl-1,7,8,9,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50409033 (CHEMBL2112735) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50093592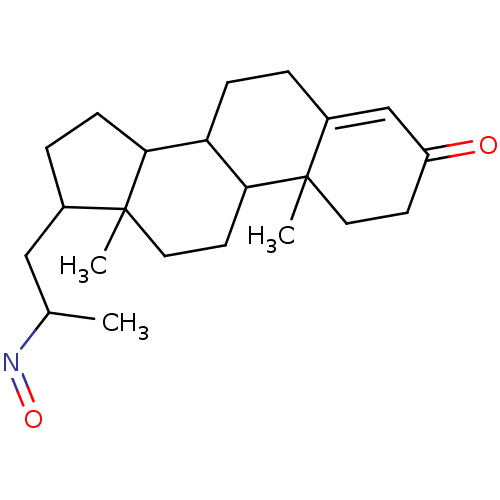 (17-(2-Hydroxyimino-propyl)-10,13-dimethyl-1,2,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8946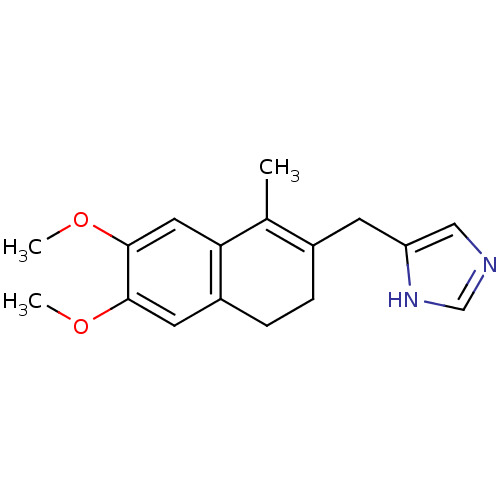 (3,4-Dihydro-2-(5-imidazolyl)-6,7-dimethoxy-1-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409032 (CHEMBL2112737) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50093590 ((10,13-Dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50409034 (CHEMBL2112731) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cells | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50093594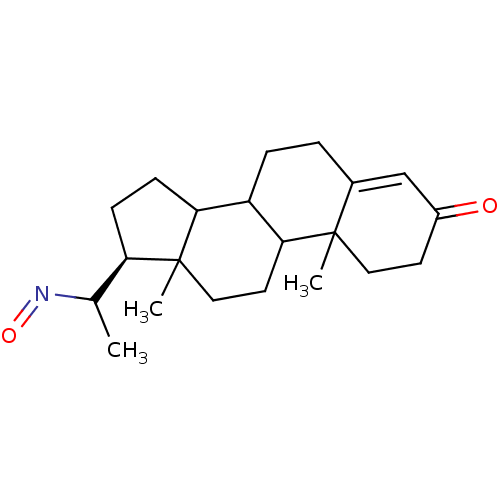 (17-(1-Hydroxyimino-ethyl)-10,13-dimethyl-1,2,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50093594 (17-(1-Hydroxyimino-ethyl)-10,13-dimethyl-1,2,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM31768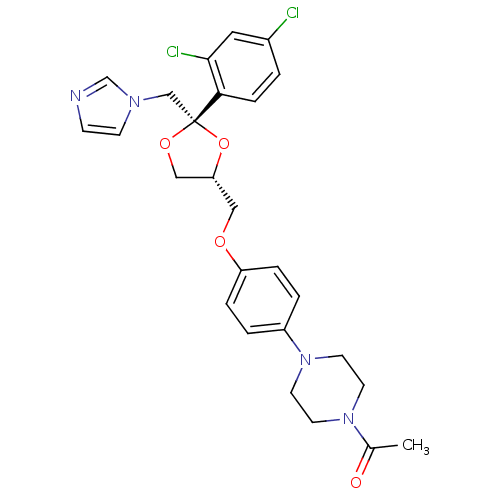 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50093598 (17-(2-Hydroxyimino-propyl)-10,13-dimethyl-1,7,8,9,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cells | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50093590 ((10,13-Dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cells | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50093592 (17-(2-Hydroxyimino-propyl)-10,13-dimethyl-1,2,6,7,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedione | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50093598 (17-(2-Hydroxyimino-propyl)-10,13-dimethyl-1,7,8,9,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedione | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8937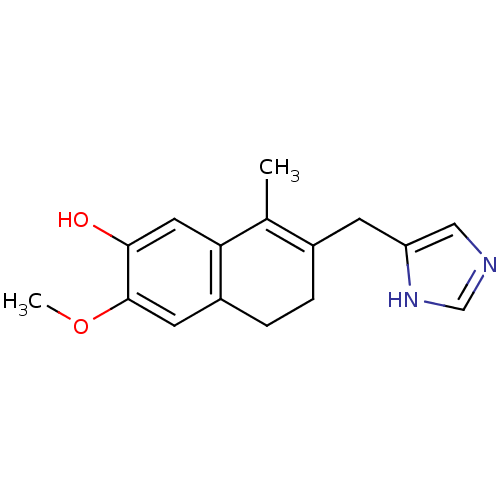 (7-(1H-imidazol-5-ylmethyl)-3-methoxy-8-methyl-5,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50093596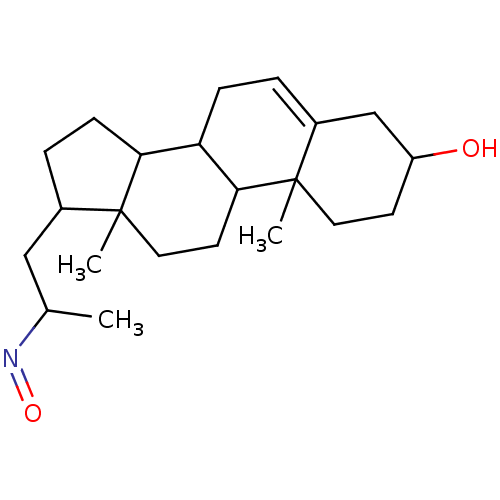 (1-(3-Hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8953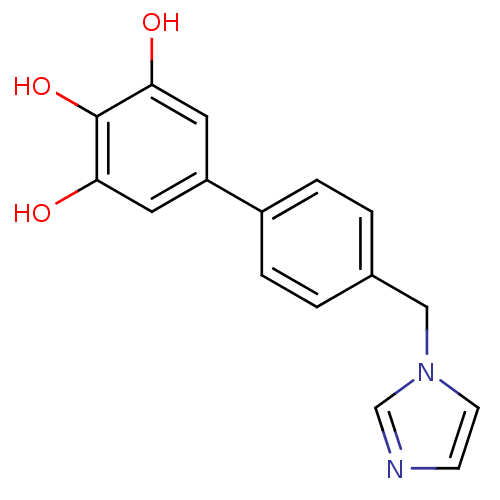 (4-Imidazol-1-ylmethyl-biphenyl-3,4,5-triol | 5-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8933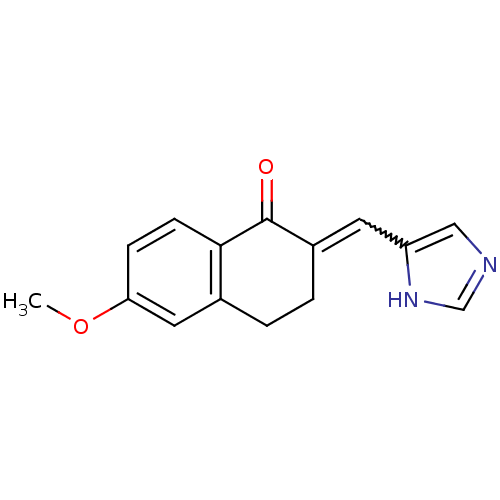 ((2E)-2-(1H-imidazol-5-ylmethylidene)-6-methoxy-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8935 (5-[(6-methoxy-1-methyl-3,4-dihydronaphthalen-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50093594 (17-(1-Hydroxyimino-ethyl)-10,13-dimethyl-1,2,6,7,8...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedione | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409031 (CHEMBL2112746) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 17 of human testicular microsomes at 25 uM progesterone | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8937 (7-(1H-imidazol-5-ylmethyl)-3-methoxy-8-methyl-5,6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50093594 (17-(1-Hydroxyimino-ethyl)-10,13-dimethyl-1,2,6,7,8...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cells | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8951 (4-Imidazol-1-ylmethyl-biphenyl-3,4-diol | 4-[4-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50093590 ((10,13-Dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedione | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8941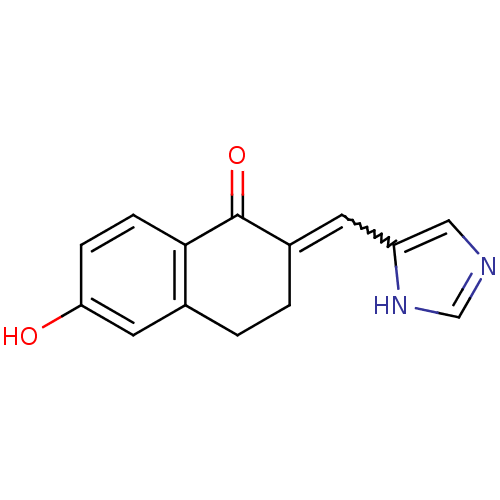 ((2E)-6-hydroxy-2-(1H-imidazol-5-ylmethylidene)-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8942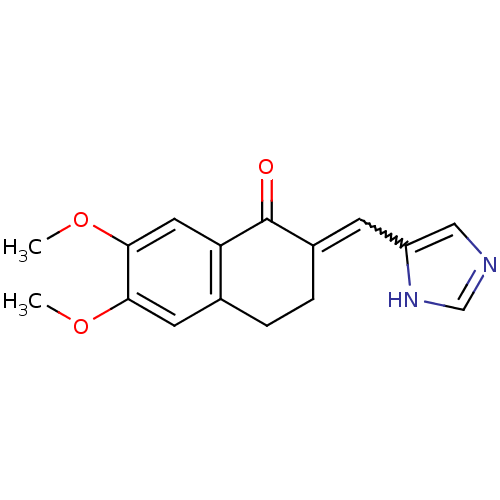 ((2E)-2-(1H-imidazol-5-ylmethylidene)-6,7-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8936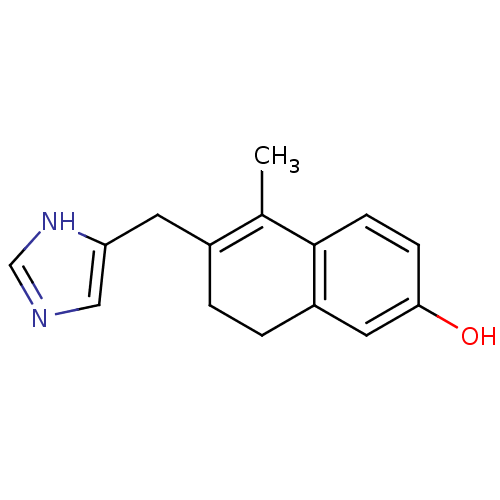 (6-(1H-imidazol-5-ylmethyl)-5-methyl-7,8-dihydronap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50093590 ((10,13-Dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cells | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50093605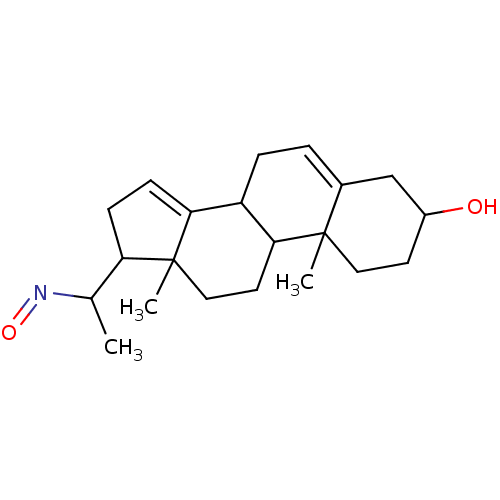 (1-(3-Hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland Curated by ChEMBL | Assay Description Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone) | J Med Chem 43: 4266-77 (2000) BindingDB Entry DOI: 10.7270/Q2VT1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |