 Found 370 hits with Last Name = 'fantacuzzi' and Initial = 'm'
Found 370 hits with Last Name = 'fantacuzzi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50595010
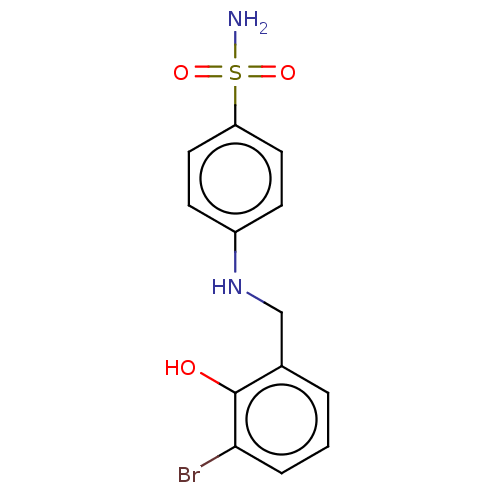
(CHEMBL5196990) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50142631
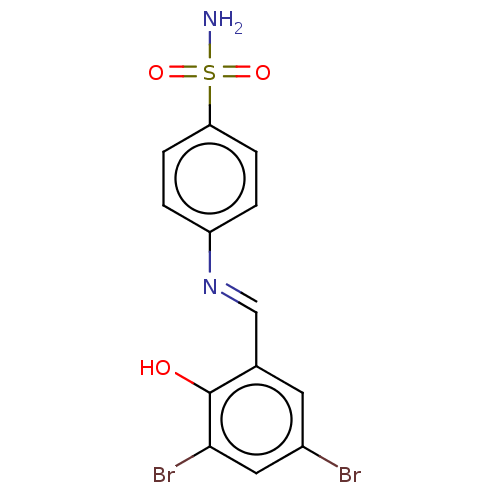
(CHEMBL3758997)Show InChI InChI=1S/C13H10Br2N2O3S/c14-9-5-8(13(18)12(15)6-9)7-17-10-1-3-11(4-2-10)21(16,19)20/h1-7,18H,(H2,16,19,20)/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50595008
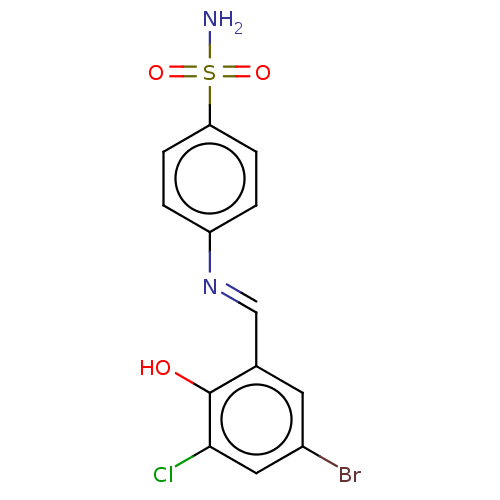
(CHEMBL5181066) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50142631

(CHEMBL3758997)Show InChI InChI=1S/C13H10Br2N2O3S/c14-9-5-8(13(18)12(15)6-9)7-17-10-1-3-11(4-2-10)21(16,19)20/h1-7,18H,(H2,16,19,20)/b17-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50595008

(CHEMBL5181066) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50595009
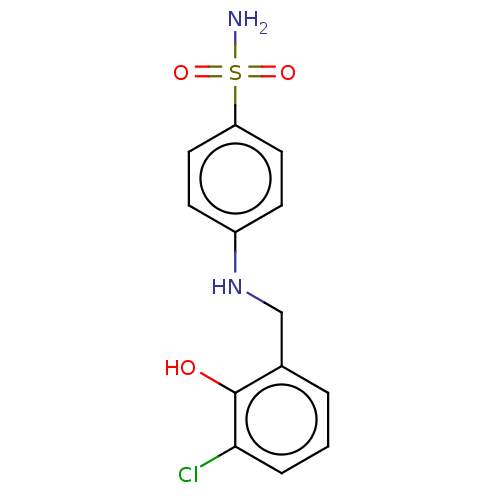
(CHEMBL5170213) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50595008

(CHEMBL5181066) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50595009

(CHEMBL5170213) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50142631

(CHEMBL3758997)Show InChI InChI=1S/C13H10Br2N2O3S/c14-9-5-8(13(18)12(15)6-9)7-17-10-1-3-11(4-2-10)21(16,19)20/h1-7,18H,(H2,16,19,20)/b17-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50595010

(CHEMBL5196990) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50142631

(CHEMBL3758997)Show InChI InChI=1S/C13H10Br2N2O3S/c14-9-5-8(13(18)12(15)6-9)7-17-10-1-3-11(4-2-10)21(16,19)20/h1-7,18H,(H2,16,19,20)/b17-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human MCF-7aro cells using [1beta-3H] androstenedione as substrate incubated for 1 hr by liquid scintillation counting met... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111815
BindingDB Entry DOI: 10.7270/Q2NC64GH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171310
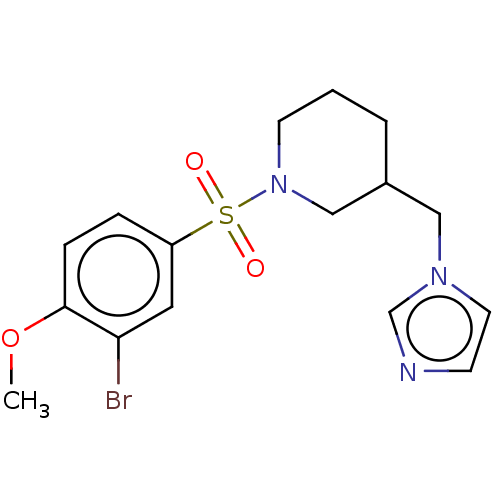
(CHEMBL3805814)Show InChI InChI=1S/C16H20BrN3O3S/c1-23-16-5-4-14(9-15(16)17)24(21,22)20-7-2-3-13(11-20)10-19-8-6-18-12-19/h4-6,8-9,12-13H,2-3,7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171231
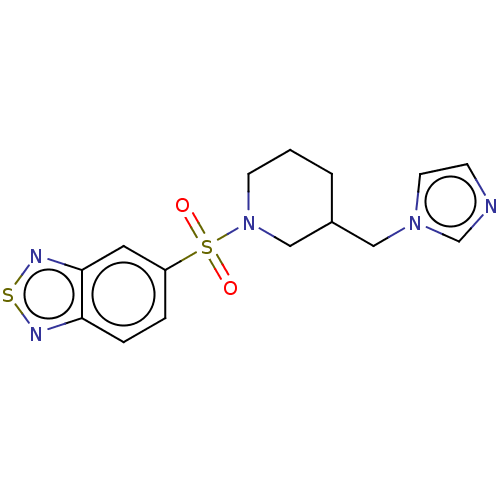
(CHEMBL3805851)Show InChI InChI=1S/C15H17N5O2S2/c21-24(22,13-3-4-14-15(8-13)18-23-17-14)20-6-1-2-12(10-20)9-19-7-5-16-11-19/h3-5,7-8,11-12H,1-2,6,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171227

(CHEMBL3806301)Show SMILES [O-][N+](=O)c1cccc(Cl)c1Oc1cccc(c1)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C21H21ClN4O5S/c22-19-7-2-8-20(26(27)28)21(19)31-17-5-1-6-18(12-17)32(29,30)25-10-3-4-16(14-25)13-24-11-9-23-15-24/h1-2,5-9,11-12,15-16H,3-4,10,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50345657
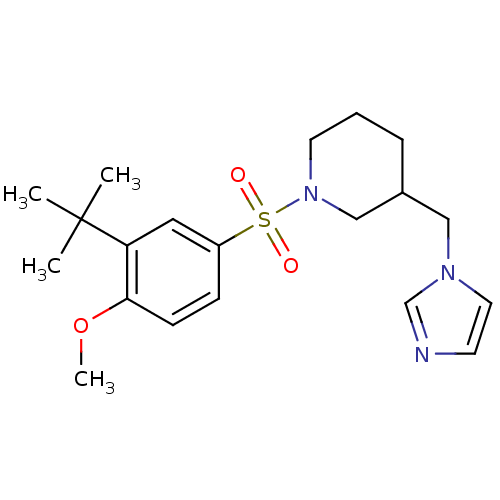
(CHEMBL1784801 | rac-3-((1H-imidazol-1-yl)methyl)-1...)Show SMILES COc1ccc(cc1C(C)(C)C)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C20H29N3O3S/c1-20(2,3)18-12-17(7-8-19(18)26-4)27(24,25)23-10-5-6-16(14-23)13-22-11-9-21-15-22/h7-9,11-12,15-16H,5-6,10,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50543107

(CHEMBL4633346)Show InChI InChI=1S/C15H14FN3O/c1-10(17)18-13-6-2-11(3-7-13)15(20)19-14-8-4-12(16)5-9-14/h2-9H,1H3,(H2,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human iNOS expressed in Escherichia coli using L-arginine as substrate preincubated for 15 mins follo... |
ACS Med Chem Lett 11: 1470-1475 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00285
BindingDB Entry DOI: 10.7270/Q2X06BMG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171229
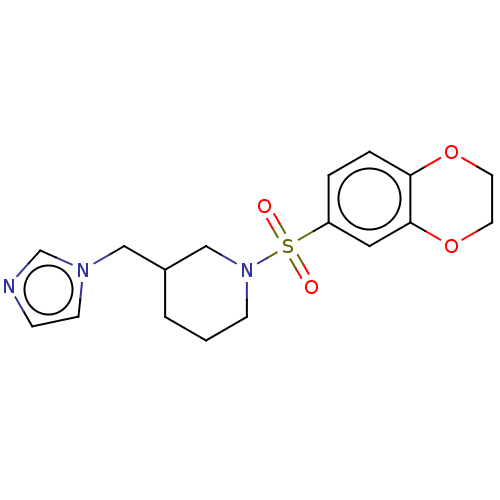
(CHEMBL3805211)Show InChI InChI=1S/C17H21N3O4S/c21-25(22,15-3-4-16-17(10-15)24-9-8-23-16)20-6-1-2-14(12-20)11-19-7-5-18-13-19/h3-5,7,10,13-14H,1-2,6,8-9,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171390

(CHEMBL3805733)Show SMILES O=S(=O)(N1CCCC(Cn2ccnc2)C1)c1ccc(OC2CCCC2)cc1 Show InChI InChI=1S/C20H27N3O3S/c24-27(25,20-9-7-19(8-10-20)26-18-5-1-2-6-18)23-12-3-4-17(15-23)14-22-13-11-21-16-22/h7-11,13,16-18H,1-6,12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171228
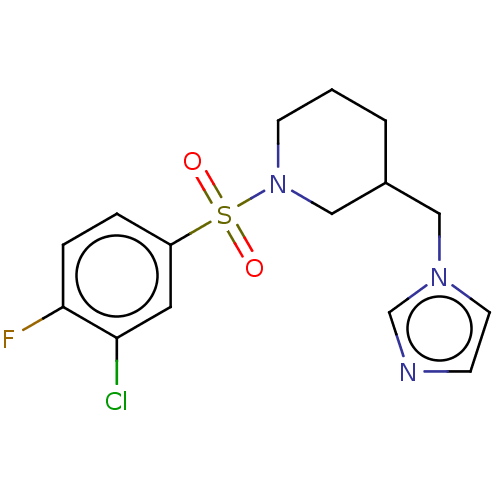
(CHEMBL3805481)Show InChI InChI=1S/C15H17ClFN3O2S/c16-14-8-13(3-4-15(14)17)23(21,22)20-6-1-2-12(10-20)9-19-7-5-18-11-19/h3-5,7-8,11-12H,1-2,6,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171226
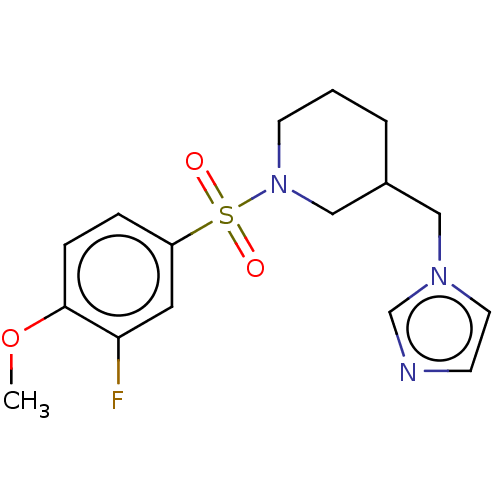
(CHEMBL3805173)Show InChI InChI=1S/C16H20FN3O3S/c1-23-16-5-4-14(9-15(16)17)24(21,22)20-7-2-3-13(11-20)10-19-8-6-18-12-19/h4-6,8-9,12-13H,2-3,7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171345
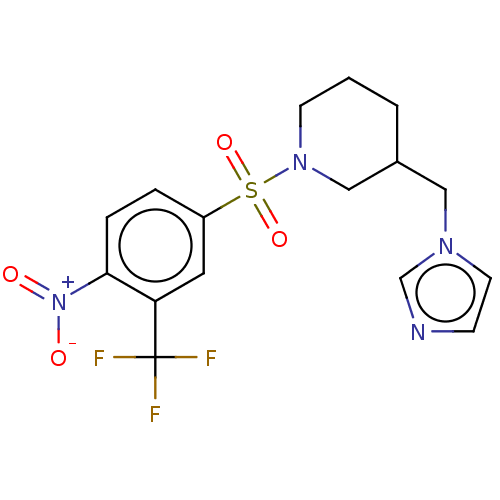
(CHEMBL3805008)Show SMILES [O-][N+](=O)c1ccc(cc1C(F)(F)F)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C16H17F3N4O4S/c17-16(18,19)14-8-13(3-4-15(14)23(24)25)28(26,27)22-6-1-2-12(10-22)9-21-7-5-20-11-21/h3-5,7-8,11-12H,1-2,6,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171348

(CHEMBL3806075)Show SMILES COc1c(C)cc(cc1C)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C18H25N3O3S/c1-14-9-17(10-15(2)18(14)24-3)25(22,23)21-7-4-5-16(12-21)11-20-8-6-19-13-20/h6,8-10,13,16H,4-5,7,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171350

(CHEMBL3805539)Show SMILES Fc1cc(ccc1OC1CCCC1)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C20H26FN3O3S/c21-19-12-18(7-8-20(19)27-17-5-1-2-6-17)28(25,26)24-10-3-4-16(14-24)13-23-11-9-22-15-23/h7-9,11-12,15-17H,1-6,10,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171208
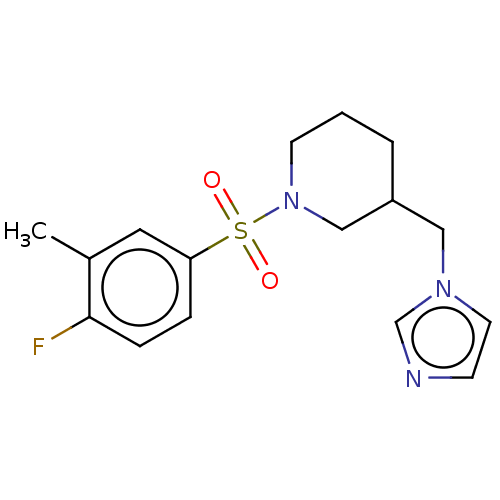
(CHEMBL3805310)Show InChI InChI=1S/C16H20FN3O2S/c1-13-9-15(4-5-16(13)17)23(21,22)20-7-2-3-14(11-20)10-19-8-6-18-12-19/h4-6,8-9,12,14H,2-3,7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171308
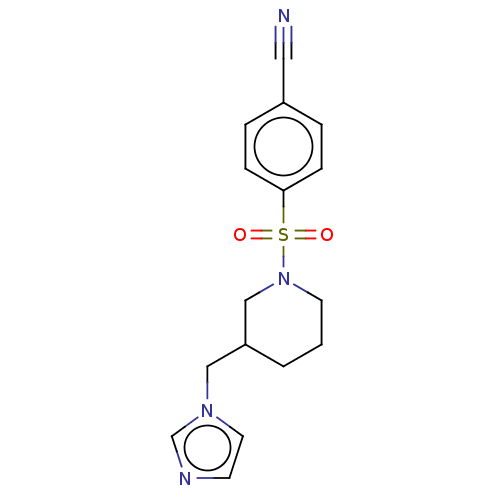
(CHEMBL3806046)Show InChI InChI=1S/C16H18N4O2S/c17-10-14-3-5-16(6-4-14)23(21,22)20-8-1-2-15(12-20)11-19-9-7-18-13-19/h3-7,9,13,15H,1-2,8,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171311
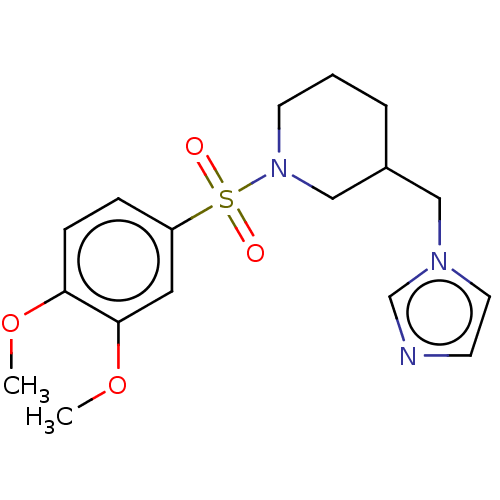
(CHEMBL3805149)Show InChI InChI=1S/C17H23N3O4S/c1-23-16-6-5-15(10-17(16)24-2)25(21,22)20-8-3-4-14(12-20)11-19-9-7-18-13-19/h5-7,9-10,13-14H,3-4,8,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171307
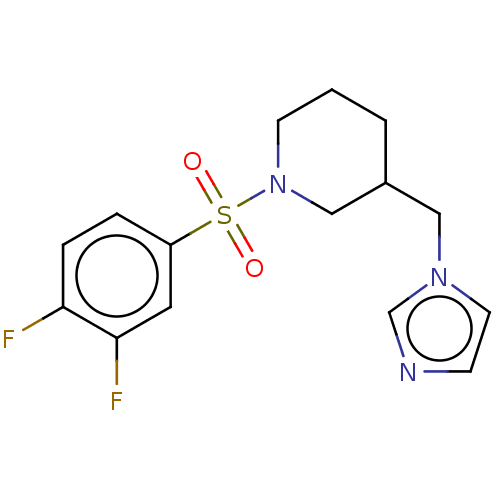
(CHEMBL3806159)Show InChI InChI=1S/C15H17F2N3O2S/c16-14-4-3-13(8-15(14)17)23(21,22)20-6-1-2-12(10-20)9-19-7-5-18-11-19/h3-5,7-8,11-12H,1-2,6,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM10989
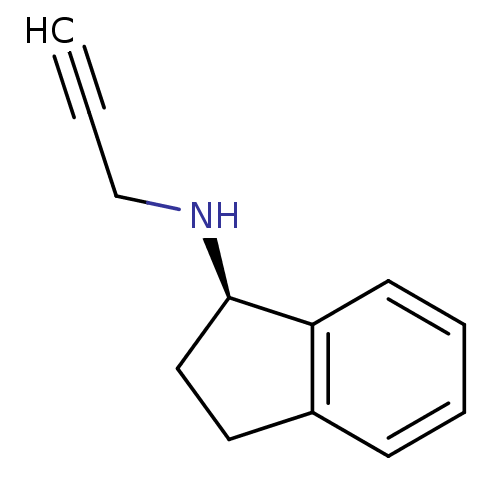
((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...)Show InChI InChI=1S/C12H13N/c1-2-9-13-12-8-7-10-5-3-4-6-11(10)12/h1,3-6,12-13H,7-9H2/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171346
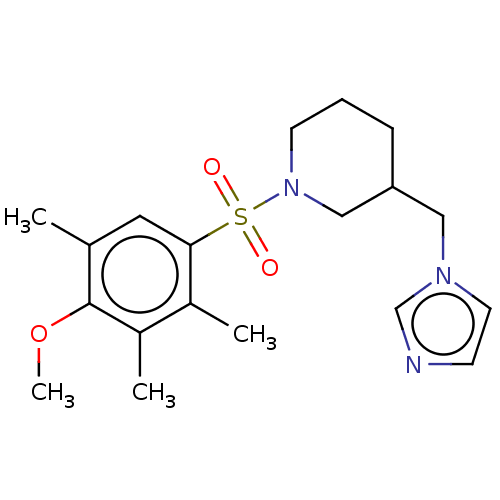
(CHEMBL3805388)Show SMILES COc1c(C)cc(c(C)c1C)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C19H27N3O3S/c1-14-10-18(15(2)16(3)19(14)25-4)26(23,24)22-8-5-6-17(12-22)11-21-9-7-20-13-21/h7,9-10,13,17H,5-6,8,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171309
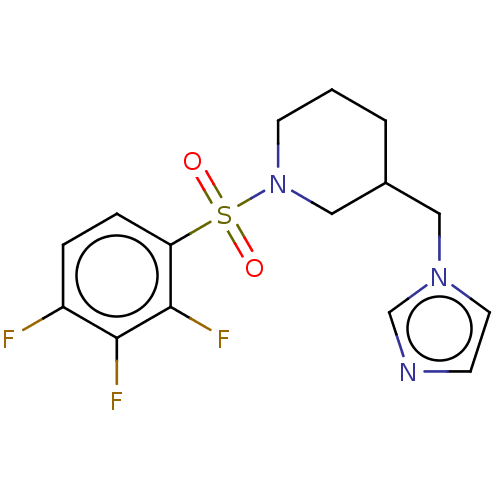
(CHEMBL3805839)Show InChI InChI=1S/C15H16F3N3O2S/c16-12-3-4-13(15(18)14(12)17)24(22,23)21-6-1-2-11(9-21)8-20-7-5-19-10-20/h3-5,7,10-11H,1-2,6,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171344

(CHEMBL3805657)Show SMILES CS(=O)(=O)c1ccc(cc1)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C16H21N3O4S2/c1-24(20,21)15-4-6-16(7-5-15)25(22,23)19-9-2-3-14(12-19)11-18-10-8-17-13-18/h4-8,10,13-14H,2-3,9,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171342
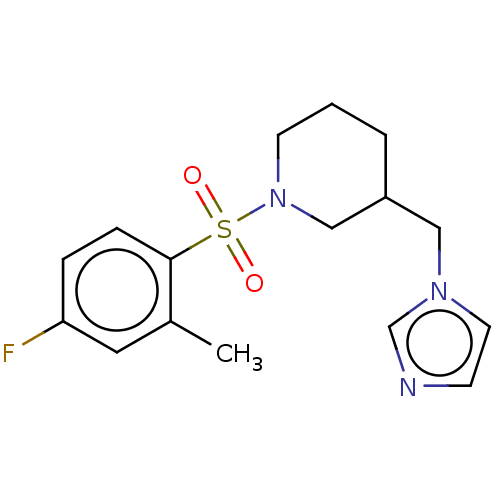
(CHEMBL3806185)Show InChI InChI=1S/C16H20FN3O2S/c1-13-9-15(17)4-5-16(13)23(21,22)20-7-2-3-14(11-20)10-19-8-6-18-12-19/h4-6,8-9,12,14H,2-3,7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171312
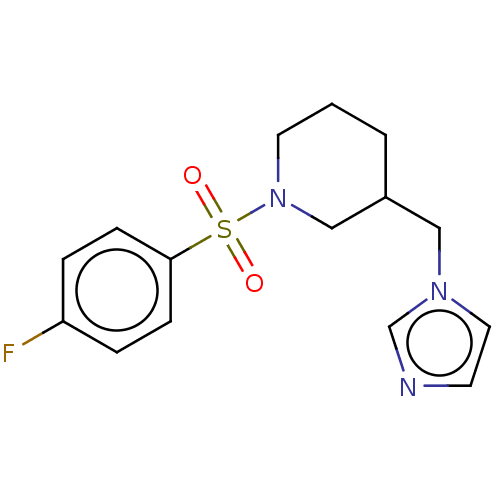
(CHEMBL3805048)Show InChI InChI=1S/C15H18FN3O2S/c16-14-3-5-15(6-4-14)22(20,21)19-8-1-2-13(11-19)10-18-9-7-17-12-18/h3-7,9,12-13H,1-2,8,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171207
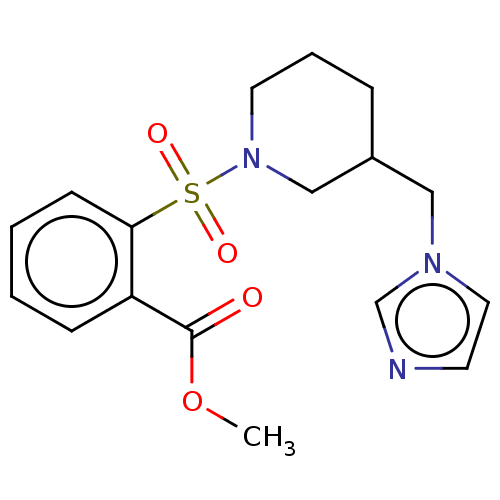
(CHEMBL3805021)Show InChI InChI=1S/C17H21N3O4S/c1-24-17(21)15-6-2-3-7-16(15)25(22,23)20-9-4-5-14(12-20)11-19-10-8-18-13-19/h2-3,6-8,10,13-14H,4-5,9,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171230

(CHEMBL3806151)Show InChI InChI=1S/C15H17N5O2S2/c21-24(22,14-5-1-4-13-15(14)18-23-17-13)20-7-2-3-12(10-20)9-19-8-6-16-11-19/h1,4-6,8,11-12H,2-3,7,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50401339
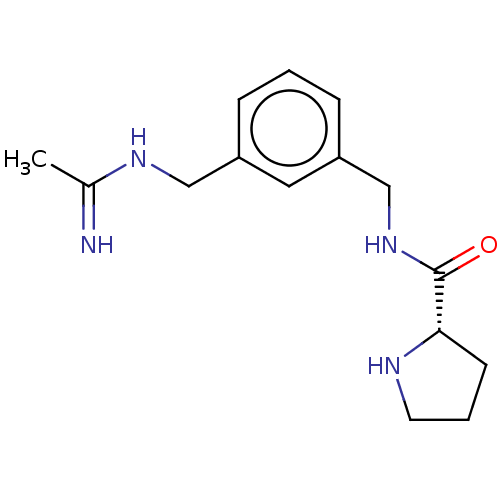
(CHEMBL4161447)Show SMILES Cl.Cl.CC(=N)NCc1cccc(CNC(=O)[C@@H]2CCCN2)c1 |r| Show InChI InChI=1S/C15H22N4O.2ClH/c1-11(16)18-9-12-4-2-5-13(8-12)10-19-15(20)14-6-3-7-17-14;;/h2,4-5,8,14,17H,3,6-7,9-10H2,1H3,(H2,16,18)(H,19,20);2*1H/t14-;;/m0../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... |
Eur J Med Chem 152: 53-64 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.027
BindingDB Entry DOI: 10.7270/Q2C2500M |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171349

(CHEMBL3805820)Show SMILES COc1ccc(c(C)c1C)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C18H25N3O3S/c1-14-15(2)18(7-6-17(14)24-3)25(22,23)21-9-4-5-16(12-21)11-20-10-8-19-13-20/h6-8,10,13,16H,4-5,9,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171306

(CHEMBL3806282)Show InChI InChI=1S/C16H17ClN4O2S/c17-15-8-13(9-18)3-4-16(15)24(22,23)21-6-1-2-14(11-21)10-20-7-5-19-12-20/h3-5,7-8,12,14H,1-2,6,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50226527

(CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human iNOS expressed in Escherichia coli using L-arginine as substrate preincubated for 15 mins follo... |
ACS Med Chem Lett 11: 1470-1475 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00285
BindingDB Entry DOI: 10.7270/Q2X06BMG |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50226527

(CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS using [3H]L-arginine substrate assessed as formation of [3H]L-citruline by liquid scintillation counting analysi... |
ACS Med Chem Lett 6: 635-40 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00149
BindingDB Entry DOI: 10.7270/Q2C53NKC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50095303

(CHEMBL3589092)Show InChI InChI=1S/C17H20N4O2/c1-13(18)20-11-15-6-4-5-14(9-15)10-19-12-16-7-2-3-8-17(16)21(22)23/h2-9,19H,10-12H2,1H3,(H2,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS using [3H]L-arginine substrate assessed as formation of [3H]L-citruline by liquid scintillation counting analysi... |
ACS Med Chem Lett 6: 635-40 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00149
BindingDB Entry DOI: 10.7270/Q2C53NKC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50226527

(CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... |
Eur J Med Chem 152: 53-64 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.027
BindingDB Entry DOI: 10.7270/Q2C2500M |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171343
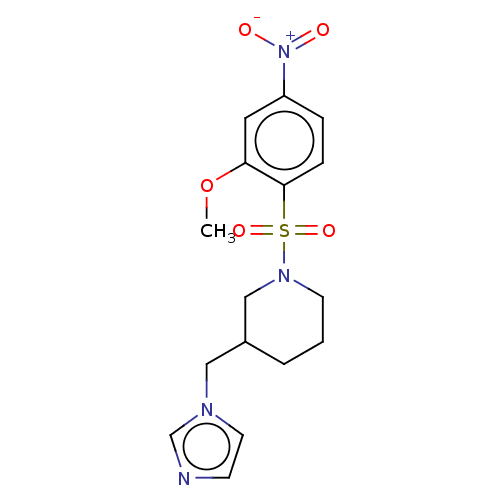
(CHEMBL3804993)Show SMILES COc1cc(ccc1S(=O)(=O)N1CCCC(Cn2ccnc2)C1)[N+]([O-])=O Show InChI InChI=1S/C16H20N4O5S/c1-25-15-9-14(20(21)22)4-5-16(15)26(23,24)19-7-2-3-13(11-19)10-18-8-6-17-12-18/h4-6,8-9,12-13H,2-3,7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171300

(CHEMBL3805678)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C17H23N3O4S/c1-23-15-5-6-17(16(10-15)24-2)25(21,22)20-8-3-4-14(12-20)11-19-9-7-18-13-19/h5-7,9-10,13-14H,3-4,8,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data