

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50230576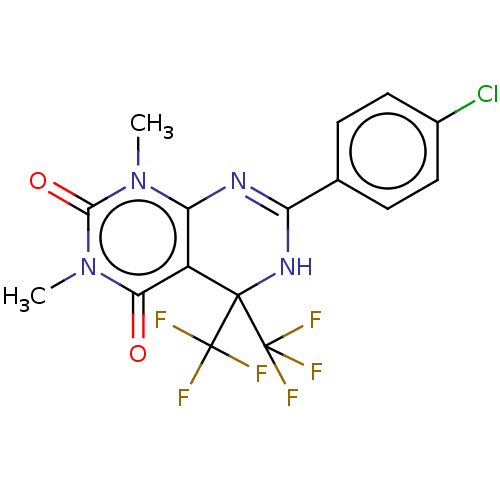 (CHEMBL1341270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50230575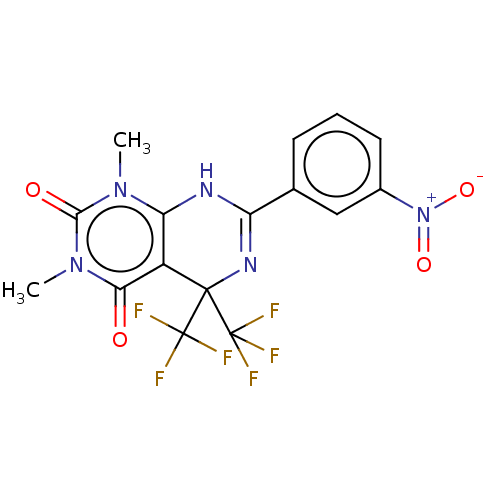 (CHEMBL4079193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 603 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230573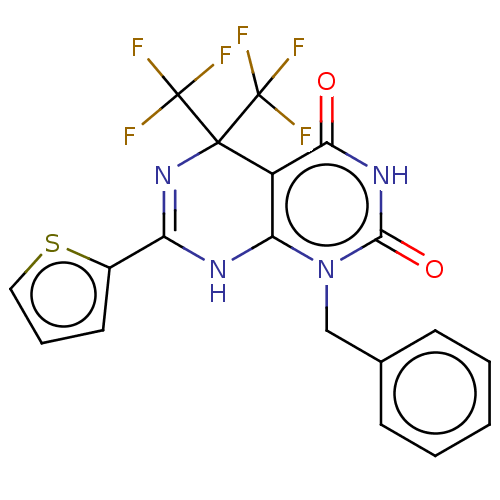 (CHEMBL4068337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230573 (CHEMBL4068337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230573 (CHEMBL4068337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230573 (CHEMBL4068337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50230577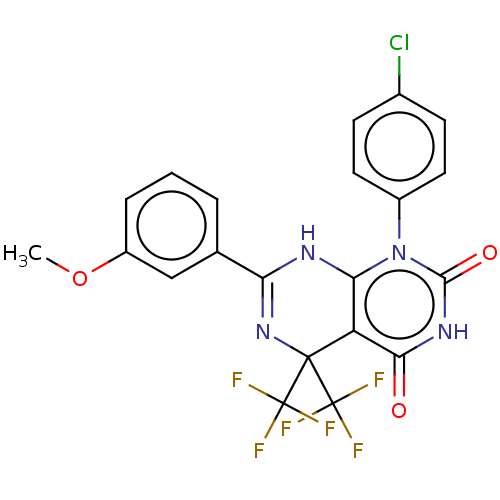 (CHEMBL4095693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230577 (CHEMBL4095693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230577 (CHEMBL4095693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230577 (CHEMBL4095693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230577 (CHEMBL4095693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230574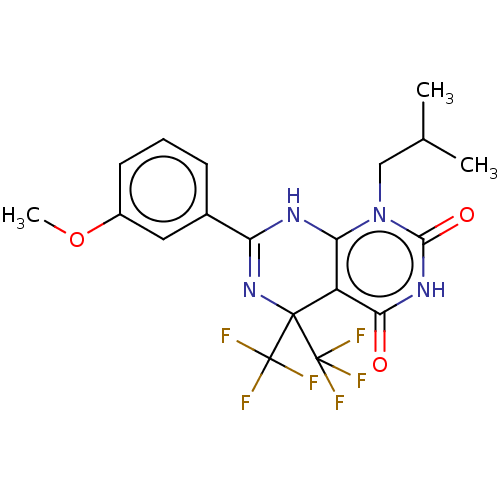 (CHEMBL4103452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230574 (CHEMBL4103452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230574 (CHEMBL4103452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230574 (CHEMBL4103452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50230577 (CHEMBL4095693) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 15 mins followed by NADPH addition measured after... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50230577 (CHEMBL4095693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 15 mins followed by NADPH addition measured after 8 mins... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230578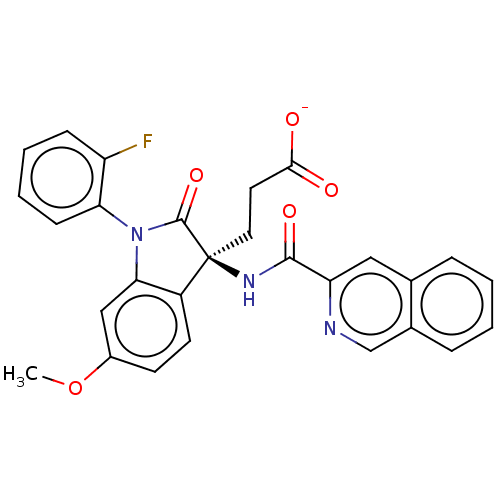 (CHEMBL1628665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at GLP-1R (unknown origin) | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 15 mins followed by NADPH addition measured after... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 15 mins followed by NADPH addition measured after 8 mins... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50230577 (CHEMBL4095693) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at glucagon receptor (unknown origin) | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at glucagon receptor (unknown origin) | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50230577 (CHEMBL4095693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 15 mins followed by NADPH addition measured after... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 15 mins followed by NADPH addition measured after 8 mins... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at glucagon receptor (unknown origin) | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||