 Found 250 hits with Last Name = 'farre-gutierrez' and Initial = 'i'
Found 250 hits with Last Name = 'farre-gutierrez' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311748
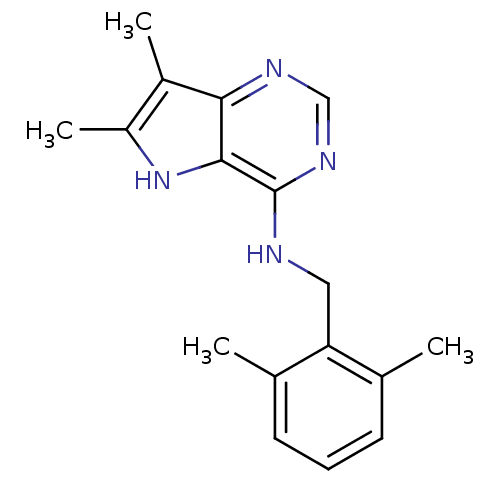
(CHEMBL1081504 | N-(2,6-dimethylbenzyl)-6,7-dimethy...)Show InChI InChI=1S/C17H20N4/c1-10-6-5-7-11(2)14(10)8-18-17-16-15(19-9-20-17)12(3)13(4)21-16/h5-7,9,21H,8H2,1-4H3,(H,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50311760
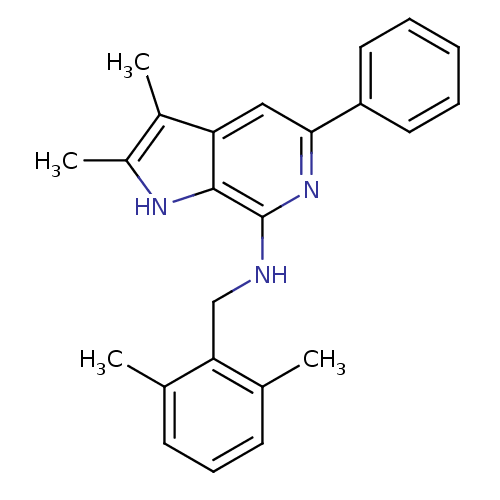
(CHEMBL1080473 | N-(2,6-dimethylbenzyl)-2,3-dimethy...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)-c1ccccc1 Show InChI InChI=1S/C24H25N3/c1-15-9-8-10-16(2)21(15)14-25-24-23-20(17(3)18(4)26-23)13-22(27-24)19-11-6-5-7-12-19/h5-13,26H,14H2,1-4H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50268742

(CHEMBL523752 | N-(2-ethyl-6-methylbenzyl)-2,3-dime...)Show InChI InChI=1S/C19H23N3/c1-5-16-9-6-8-13(2)17(16)12-20-18-10-7-11-22-15(4)14(3)21-19(18)22/h6-11,20H,5,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311761

(1-(1-allyl-7-(2,6-dimethylbenzylamino)-2,3-dimethy...)Show SMILES Cc1c(C)c2cc(nc(NCc3c(C)cccc3C)c2n1CC=C)-n1ccccc1=O Show InChI InChI=1S/C26H28N4O/c1-6-13-29-20(5)19(4)21-15-23(30-14-8-7-12-24(30)31)28-26(25(21)29)27-16-22-17(2)10-9-11-18(22)3/h6-12,14-15H,1,13,16H2,2-5H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311760

(CHEMBL1080473 | N-(2,6-dimethylbenzyl)-2,3-dimethy...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)-c1ccccc1 Show InChI InChI=1S/C24H25N3/c1-15-9-8-10-16(2)21(15)14-25-24-23-20(17(3)18(4)26-23)13-22(27-24)19-11-6-5-7-12-19/h5-13,26H,14H2,1-4H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50268782
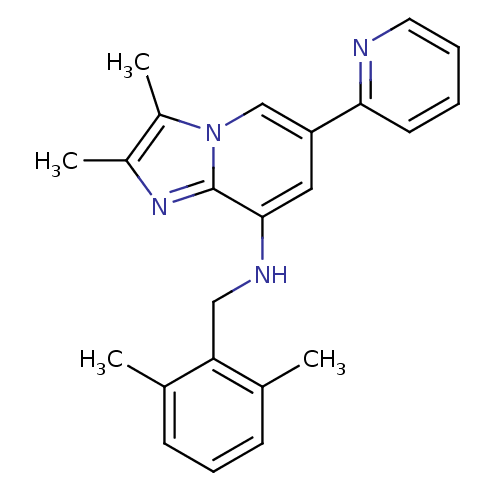
(CHEMBL447822 | N-(2,6-dimethylbenzyl)-2,3-dimethyl...)Show InChI InChI=1S/C23H24N4/c1-15-8-7-9-16(2)20(15)13-25-22-12-19(21-10-5-6-11-24-21)14-27-18(4)17(3)26-23(22)27/h5-12,14,25H,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50269080

((S)-2,3-dimethyl-9-phenyl-6-(1H-1,2,4-triazol-1-yl...)Show SMILES Cc1nc2c3N[C@@H](CCc3c(cn2c1C)-n1cncn1)c1ccccc1 |r| Show InChI InChI=1S/C20H20N6/c1-13-14(2)25-10-18(26-12-21-11-22-26)16-8-9-17(15-6-4-3-5-7-15)24-19(16)20(25)23-13/h3-7,10-12,17,24H,8-9H2,1-2H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50311754
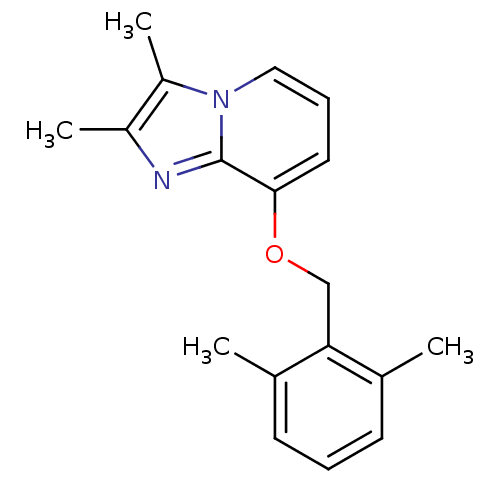
(8-(2,6-dimethylbenzyloxy)-2,3-dimethylimidazo[1,2-...)Show InChI InChI=1S/C18H20N2O/c1-12-7-5-8-13(2)16(12)11-21-17-9-6-10-20-15(4)14(3)19-18(17)20/h5-10H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311756
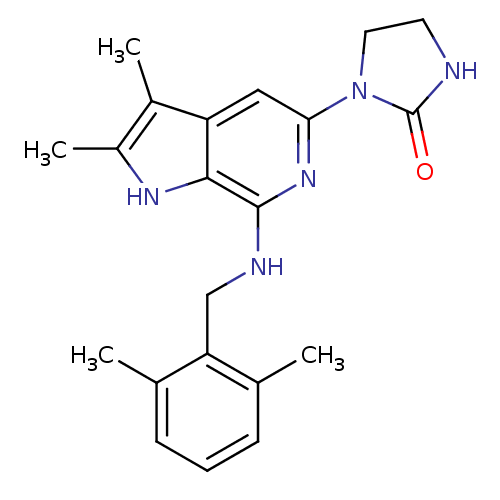
(1-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)N1CCNC1=O Show InChI InChI=1S/C21H25N5O/c1-12-6-5-7-13(2)17(12)11-23-20-19-16(14(3)15(4)24-19)10-18(25-20)26-9-8-22-21(26)27/h5-7,10,24H,8-9,11H2,1-4H3,(H,22,27)(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311763

(1-(7-(2,6-dimethylbenzylamino)-1-ethyl-2,3-dimethy...)Show SMILES CCn1c(C)c(C)c2cc(nc(NCc3c(C)cccc3C)c12)-n1ccccc1=O Show InChI InChI=1S/C25H28N4O/c1-6-28-19(5)18(4)20-14-22(29-13-8-7-12-23(29)30)27-25(24(20)28)26-15-21-16(2)10-9-11-17(21)3/h7-14H,6,15H2,1-5H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311746

(7-(4-fluorobenzyloxy)-2,3-dimethyl-1-(((1S,2S)-2-m...)Show SMILES C[C@H]1C[C@@H]1Cn1c(C)c(C)c2cnnc(OCc3ccc(F)cc3)c12 |r| Show InChI InChI=1S/C20H22FN3O/c1-12-8-16(12)10-24-14(3)13(2)18-9-22-23-20(19(18)24)25-11-15-4-6-17(21)7-5-15/h4-7,9,12,16H,8,10-11H2,1-3H3/t12-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50268780

(CHEMBL453246 | N-(2,6-dimethylbenzyl)-2,3-dimethyl...)Show InChI InChI=1S/C21H23N5/c1-14-7-5-8-15(2)19(14)12-22-20-11-18(26-10-6-9-23-26)13-25-17(4)16(3)24-21(20)25/h5-11,13,22H,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311759

(1-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)N1CCCC1=O Show InChI InChI=1S/C22H26N4O/c1-13-7-5-8-14(2)18(13)12-23-22-21-17(15(3)16(4)24-21)11-19(25-22)26-10-6-9-20(26)27/h5,7-8,11,24H,6,9-10,12H2,1-4H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50268742

(CHEMBL523752 | N-(2-ethyl-6-methylbenzyl)-2,3-dime...)Show InChI InChI=1S/C19H23N3/c1-5-16-9-6-8-13(2)17(16)12-20-18-10-7-11-22-15(4)14(3)21-19(18)22/h6-11,20H,5,12H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50268983
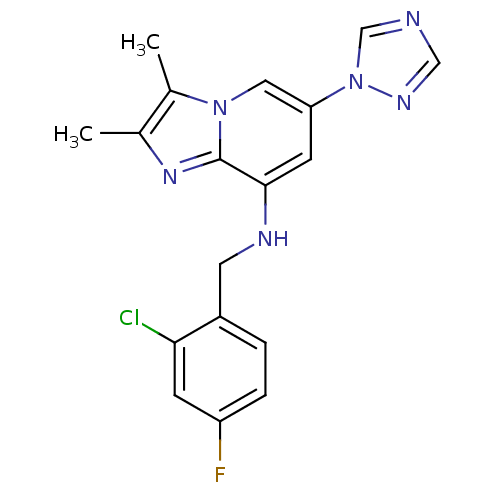
(CHEMBL526841 | N-(2-chloro-4-fluorobenzyl)-2,3-dim...)Show InChI InChI=1S/C18H16ClFN6/c1-11-12(2)25-8-15(26-10-21-9-23-26)6-17(18(25)24-11)22-7-13-3-4-14(20)5-16(13)19/h3-6,8-10,22H,7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50311746

(7-(4-fluorobenzyloxy)-2,3-dimethyl-1-(((1S,2S)-2-m...)Show SMILES C[C@H]1C[C@@H]1Cn1c(C)c(C)c2cnnc(OCc3ccc(F)cc3)c12 |r| Show InChI InChI=1S/C20H22FN3O/c1-12-8-16(12)10-24-14(3)13(2)18-9-22-23-20(19(18)24)25-11-15-4-6-17(21)7-5-15/h4-7,9,12,16H,8,10-11H2,1-3H3/t12-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268782

(CHEMBL447822 | N-(2,6-dimethylbenzyl)-2,3-dimethyl...)Show InChI InChI=1S/C23H24N4/c1-15-8-7-9-16(2)20(15)13-25-22-12-19(21-10-5-6-11-24-21)14-27-18(4)17(3)26-23(22)27/h5-12,14,25H,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50268887

(CHEMBL496426 | N-(2,6-dimethylbenzyl)-6-(furan-2-y...)Show InChI InChI=1S/C22H23N3O/c1-14-7-5-8-15(2)19(14)12-23-20-11-18(21-9-6-10-26-21)13-25-17(4)16(3)24-22(20)25/h5-11,13,23H,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311757

(6-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)-c1cccc(=O)[nH]1 Show InChI InChI=1S/C23H24N4O/c1-13-7-5-8-14(2)18(13)12-24-23-22-17(15(3)16(4)25-22)11-20(27-23)19-9-6-10-21(28)26-19/h5-11,25H,12H2,1-4H3,(H,24,27)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311759

(1-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)N1CCCC1=O Show InChI InChI=1S/C22H26N4O/c1-13-7-5-8-14(2)18(13)12-23-22-21-17(15(3)16(4)24-21)11-19(25-22)26-10-6-9-20(26)27/h5,7-8,11,24H,6,9-10,12H2,1-4H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268887

(CHEMBL496426 | N-(2,6-dimethylbenzyl)-6-(furan-2-y...)Show InChI InChI=1S/C22H23N3O/c1-14-7-5-8-15(2)19(14)12-23-20-11-18(21-9-6-10-26-21)13-25-17(4)16(3)24-22(20)25/h5-11,13,23H,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50268742

(CHEMBL523752 | N-(2-ethyl-6-methylbenzyl)-2,3-dime...)Show InChI InChI=1S/C19H23N3/c1-5-16-9-6-8-13(2)17(16)12-20-18-10-7-11-22-15(4)14(3)21-19(18)22/h6-11,20H,5,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50268742

(CHEMBL523752 | N-(2-ethyl-6-methylbenzyl)-2,3-dime...)Show InChI InChI=1S/C19H23N3/c1-5-16-9-6-8-13(2)17(16)12-20-18-10-7-11-22-15(4)14(3)21-19(18)22/h6-11,20H,5,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311747

(CHEMBL1080717 | N-(2,6-dimethylbenzyl)-6,7-dimethy...)Show InChI InChI=1S/C18H21N3/c1-12-6-5-7-13(2)16(12)11-20-18-17-10-14(3)15(4)21(17)9-8-19-18/h5-10H,11H2,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using 7-benzyloxyquinoline substrate |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311756

(1-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)N1CCNC1=O Show InChI InChI=1S/C21H25N5O/c1-12-6-5-7-13(2)17(12)11-23-20-19-16(14(3)15(4)24-19)10-18(25-20)26-9-8-22-21(26)27/h5-7,10,24H,8-9,11H2,1-4H3,(H,22,27)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using 7-benzyloxyquinoline substrate |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311756

(1-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)N1CCNC1=O Show InChI InChI=1S/C21H25N5O/c1-12-6-5-7-13(2)17(12)11-23-20-19-16(14(3)15(4)24-19)10-18(25-20)26-9-8-22-21(26)27/h5-7,10,24H,8-9,11H2,1-4H3,(H,22,27)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268743
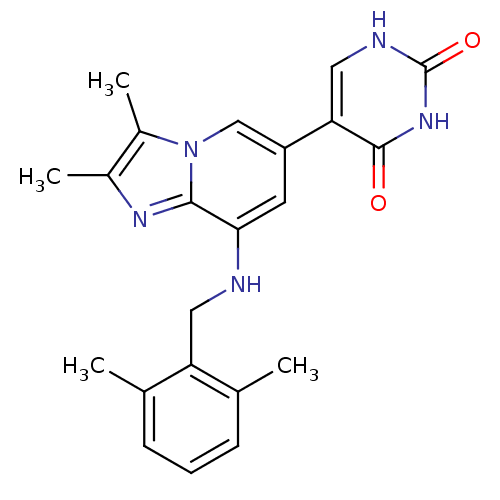
(5-(8-(2,6-dimethylbenzylamino)-2,3-dimethylimidazo...)Show SMILES Cc1nc2c(NCc3c(C)cccc3C)cc(cn2c1C)-c1c[nH]c(=O)[nH]c1=O Show InChI InChI=1S/C22H23N5O2/c1-12-6-5-7-13(2)17(12)9-23-19-8-16(18-10-24-22(29)26-21(18)28)11-27-15(4)14(3)25-20(19)27/h5-8,10-11,23H,9H2,1-4H3,(H2,24,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50268782

(CHEMBL447822 | N-(2,6-dimethylbenzyl)-2,3-dimethyl...)Show InChI InChI=1S/C23H24N4/c1-15-8-7-9-16(2)20(15)13-25-22-12-19(21-10-5-6-11-24-21)14-27-18(4)17(3)26-23(22)27/h5-12,14,25H,13H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268887

(CHEMBL496426 | N-(2,6-dimethylbenzyl)-6-(furan-2-y...)Show InChI InChI=1S/C22H23N3O/c1-14-7-5-8-15(2)19(14)12-23-20-11-18(21-9-6-10-26-21)13-25-17(4)16(3)24-22(20)25/h5-11,13,23H,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268782

(CHEMBL447822 | N-(2,6-dimethylbenzyl)-2,3-dimethyl...)Show InChI InChI=1S/C23H24N4/c1-15-8-7-9-16(2)20(15)13-25-22-12-19(21-10-5-6-11-24-21)14-27-18(4)17(3)26-23(22)27/h5-12,14,25H,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50311750

(CHEMBL1076210 | N-(2,6-dimethylbenzyl)-2,3-dimethy...)Show InChI InChI=1S/C18H21N3/c1-11-6-5-7-12(2)16(11)10-20-18-17-15(8-9-19-18)13(3)14(4)21-17/h5-9,21H,10H2,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311759

(1-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)N1CCCC1=O Show InChI InChI=1S/C22H26N4O/c1-13-7-5-8-14(2)18(13)12-23-22-21-17(15(3)16(4)24-21)11-19(25-22)26-10-6-9-20(26)27/h5,7-8,11,24H,6,9-10,12H2,1-4H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using 7-benzyloxyquinoline substrate |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311759

(1-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)N1CCCC1=O Show InChI InChI=1S/C22H26N4O/c1-13-7-5-8-14(2)18(13)12-23-22-21-17(15(3)16(4)24-21)11-19(25-22)26-10-6-9-20(26)27/h5,7-8,11,24H,6,9-10,12H2,1-4H3,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50008977
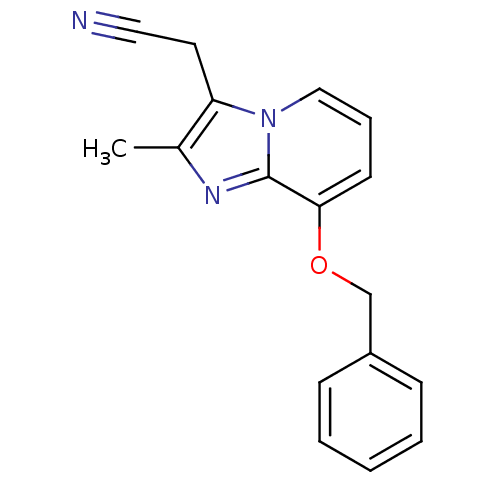
((8-Benzyloxy-2-methyl-imidazo[1,2-a]pyridin-3-yl)-...)Show InChI InChI=1S/C17H15N3O/c1-13-15(9-10-18)20-11-5-8-16(17(20)19-13)21-12-14-6-3-2-4-7-14/h2-8,11H,9,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268936
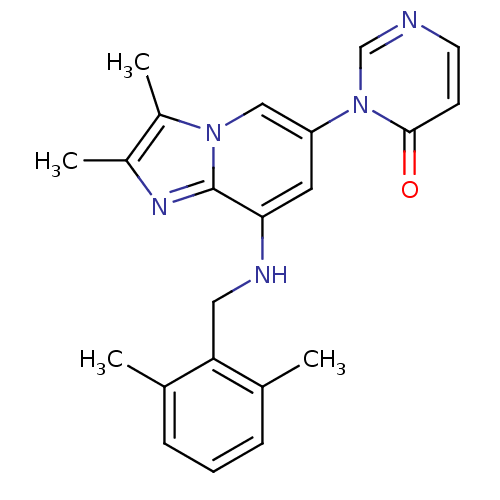
(3-(8-(2,6-dimethylbenzylamino)-2,3-dimethylimidazo...)Show SMILES Cc1nc2c(NCc3c(C)cccc3C)cc(cn2c1C)-n1cnccc1=O Show InChI InChI=1S/C22H23N5O/c1-14-6-5-7-15(2)19(14)11-24-20-10-18(27-13-23-9-8-21(27)28)12-26-17(4)16(3)25-22(20)26/h5-10,12-13,24H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268780

(CHEMBL453246 | N-(2,6-dimethylbenzyl)-2,3-dimethyl...)Show InChI InChI=1S/C21H23N5/c1-14-7-5-8-15(2)19(14)12-22-20-11-18(26-10-6-9-23-26)13-25-17(4)16(3)24-21(20)25/h5-11,13,22H,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50311753
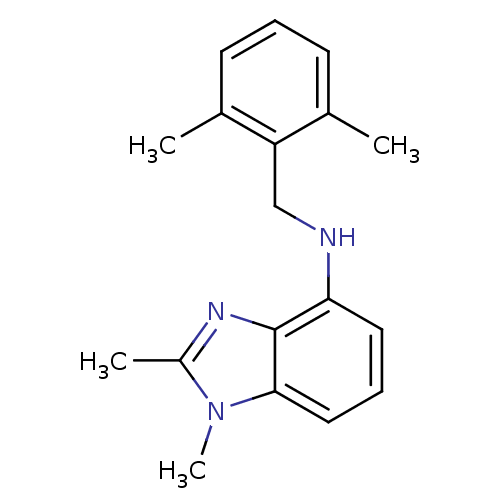
(CHEMBL1081505 | N-(2,6-dimethylbenzyl)-1,2-dimethy...)Show InChI InChI=1S/C18H21N3/c1-12-7-5-8-13(2)15(12)11-19-16-9-6-10-17-18(16)20-14(3)21(17)4/h5-10,19H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50268887

(CHEMBL496426 | N-(2,6-dimethylbenzyl)-6-(furan-2-y...)Show InChI InChI=1S/C22H23N3O/c1-14-7-5-8-15(2)19(14)12-23-20-11-18(21-9-6-10-26-21)13-25-17(4)16(3)24-22(20)25/h5-11,13,23H,12H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311757

(6-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)-c1cccc(=O)[nH]1 Show InChI InChI=1S/C23H24N4O/c1-13-7-5-8-14(2)18(13)12-24-23-22-17(15(3)16(4)25-22)11-20(27-23)19-9-6-10-21(28)26-19/h5-11,25H,12H2,1-4H3,(H,24,27)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using 7-benzyloxyquinoline substrate |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311754

(8-(2,6-dimethylbenzyloxy)-2,3-dimethylimidazo[1,2-...)Show InChI InChI=1S/C18H20N2O/c1-12-7-5-8-13(2)16(12)11-21-17-9-6-10-20-15(4)14(3)19-18(17)20/h5-10H,11H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311757

(6-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)-c1cccc(=O)[nH]1 Show InChI InChI=1S/C23H24N4O/c1-13-7-5-8-14(2)18(13)12-24-23-22-17(15(3)16(4)25-22)11-20(27-23)19-9-6-10-21(28)26-19/h5-11,25H,12H2,1-4H3,(H,24,27)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311761

(1-(1-allyl-7-(2,6-dimethylbenzylamino)-2,3-dimethy...)Show SMILES Cc1c(C)c2cc(nc(NCc3c(C)cccc3C)c2n1CC=C)-n1ccccc1=O Show InChI InChI=1S/C26H28N4O/c1-6-13-29-20(5)19(4)21-15-23(30-14-8-7-12-24(30)31)28-26(25(21)29)27-16-22-17(2)10-9-11-18(22)3/h6-12,14-15H,1,13,16H2,2-5H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using diethoxyfluorescein substrate |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50269038

((S)-1-(8-(2,3-dihydro-1H-inden-1-ylamino)-2,3-dime...)Show SMILES Cc1nc2c(N[C@H]3CCc4ccccc34)cc(cn2c1C)-n1ccccc1=O |r| Show InChI InChI=1S/C23H22N4O/c1-15-16(2)27-14-18(26-12-6-5-9-22(26)28)13-21(23(27)24-15)25-20-11-10-17-7-3-4-8-19(17)20/h3-9,12-14,20,25H,10-11H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50268783

(CHEMBL496606 | N-(2,6-dimethylbenzyl)-2,3-dimethyl...)Show InChI InChI=1S/C20H22N6/c1-13-6-5-7-14(2)18(13)9-22-19-8-17(26-12-21-11-23-26)10-25-16(4)15(3)24-20(19)25/h5-8,10-12,22H,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50269080

((S)-2,3-dimethyl-9-phenyl-6-(1H-1,2,4-triazol-1-yl...)Show SMILES Cc1nc2c3N[C@@H](CCc3c(cn2c1C)-n1cncn1)c1ccccc1 |r| Show InChI InChI=1S/C20H20N6/c1-13-14(2)25-10-18(26-12-21-11-22-26)16-8-9-17(15-6-4-3-5-7-15)24-19(16)20(25)23-13/h3-7,10-12,17,24H,8-9H2,1-2H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268743

(5-(8-(2,6-dimethylbenzylamino)-2,3-dimethylimidazo...)Show SMILES Cc1nc2c(NCc3c(C)cccc3C)cc(cn2c1C)-c1c[nH]c(=O)[nH]c1=O Show InChI InChI=1S/C22H23N5O2/c1-12-6-5-7-13(2)17(12)9-23-19-8-16(18-10-24-22(29)26-21(18)28)11-27-15(4)14(3)25-20(19)27/h5-8,10-11,23H,9H2,1-4H3,(H2,24,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311756

(1-(7-(2,6-dimethylbenzylamino)-2,3-dimethyl-1H-pyr...)Show SMILES Cc1[nH]c2c(NCc3c(C)cccc3C)nc(cc2c1C)N1CCNC1=O Show InChI InChI=1S/C21H25N5O/c1-12-6-5-7-13(2)17(12)11-23-20-19-16(14(3)15(4)24-19)10-18(25-20)26-9-8-22-21(26)27/h5-7,10,24H,8-9,11H2,1-4H3,(H,22,27)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 19: 6813-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.002
BindingDB Entry DOI: 10.7270/Q2MC9046 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268780

(CHEMBL453246 | N-(2,6-dimethylbenzyl)-2,3-dimethyl...)Show InChI InChI=1S/C21H23N5/c1-14-7-5-8-15(2)19(14)12-22-20-11-18(26-10-6-9-23-26)13-25-17(4)16(3)24-21(20)25/h5-11,13,22H,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50268981

(2,3-dimethyl-N-(2-methylbenzyl)-6-(1H-1,2,4-triazo...)Show InChI InChI=1S/C19H20N6/c1-13-6-4-5-7-16(13)9-21-18-8-17(25-12-20-11-22-25)10-24-15(3)14(2)23-19(18)24/h4-8,10-12,21H,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50268983

(CHEMBL526841 | N-(2-chloro-4-fluorobenzyl)-2,3-dim...)Show InChI InChI=1S/C18H16ClFN6/c1-11-12(2)25-8-15(26-10-21-9-23-26)6-17(18(25)24-11)22-7-13-3-4-14(20)5-16(13)19/h3-6,8-10,22H,7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Bioorg Med Chem Lett 19: 3602-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.127
BindingDB Entry DOI: 10.7270/Q2RX9BZV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data