

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50348176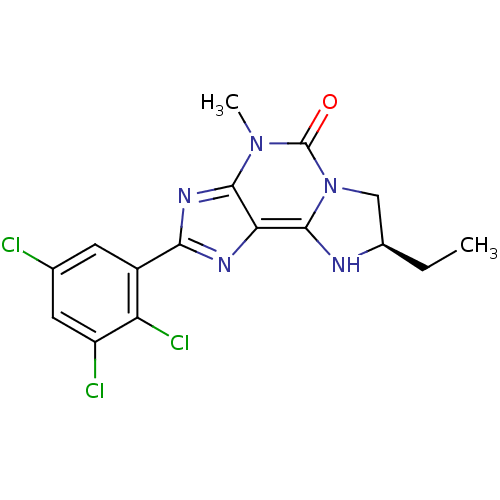 (CHEMBL1562432) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.433 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonistic activity of guinea pig Histamine H2 receptor expressed as pA2 at pH 7.4 | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50058235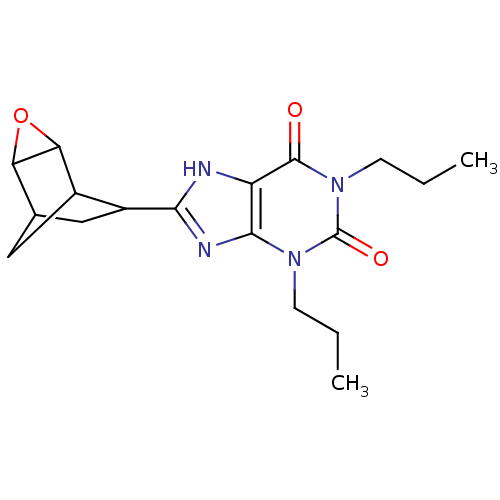 (8-(3-Oxa-tricyclo[3.2.1.0*2,4*]oct-6-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003019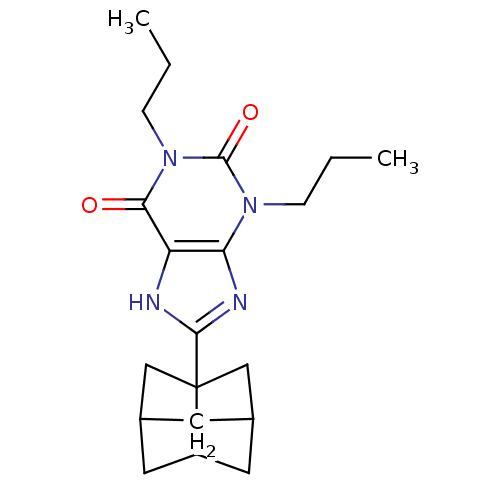 (8-(Hexahydro-2,5-methano-pentalen-3a-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086173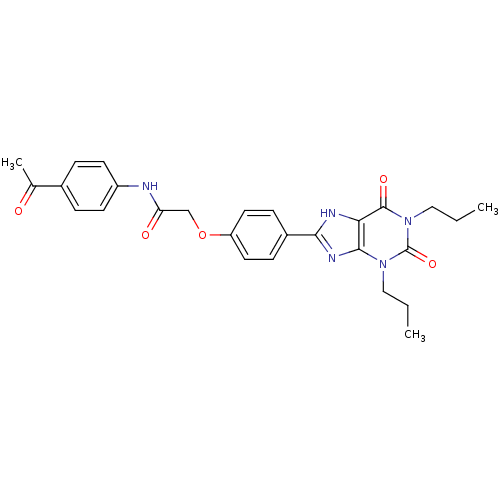 (CHEMBL17002 | N-(4-Acetyl-phenyl)-2-[4-(2,6-dioxo-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonistic activity of guinea pig Histamine H2 receptor expressed as pA2 at pH 7.8 | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086170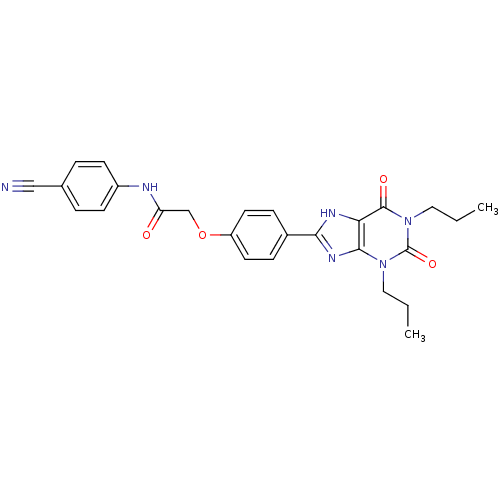 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonistic activity against Histamine H2 receptor expressed as the charge of receptor sensitivity was determined at pH 7.8 | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116370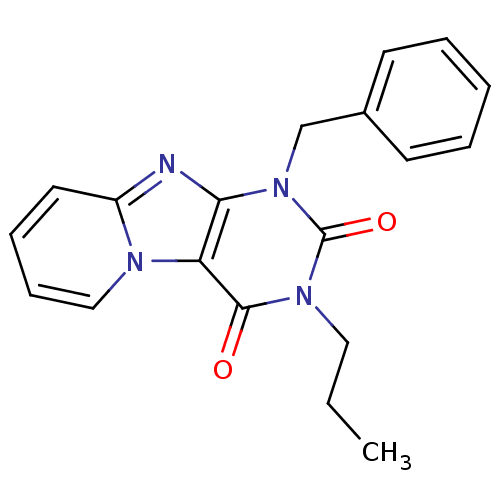 (1-Benzyl-3-propyl-1H-1,3,4b,9-tetraaza-fluorene-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonistic activity against Histamine H2 receptor expressed as the charge of receptor sensitivity was determined at pH 7.0 | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50233086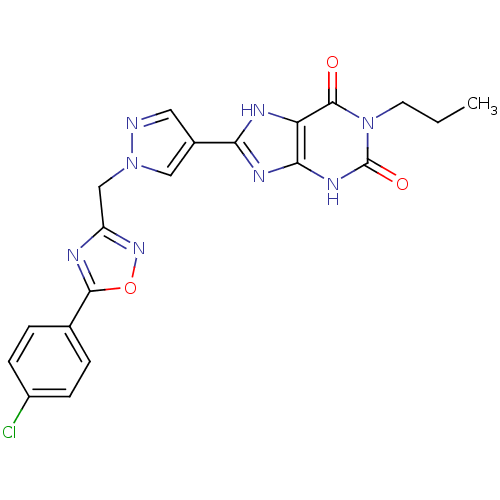 (8-(1-((5-(4-chlorophenyl)-1,2,4-oxadiazol-3-yl)met...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50020986 (CHEMBL11002 | N-(2-Dimethylamino-ethyl)-4-(2,6-dio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50227339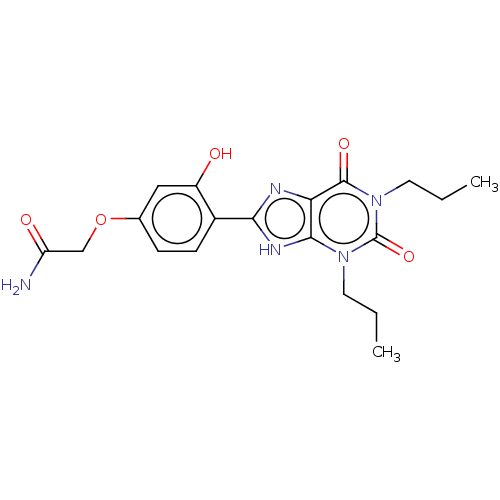 (CHEMBL158077) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50391223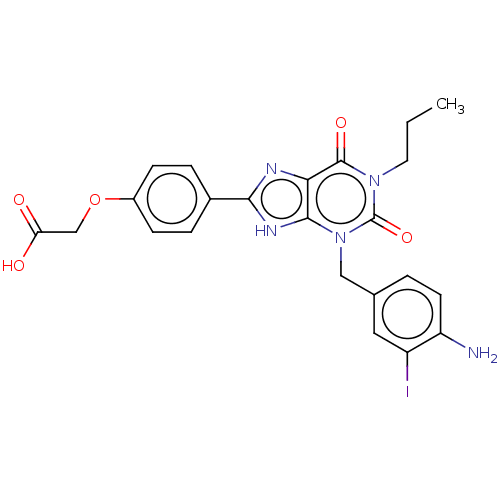 (CHEMBL355370) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50391227 (CHEMBL273671) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50042209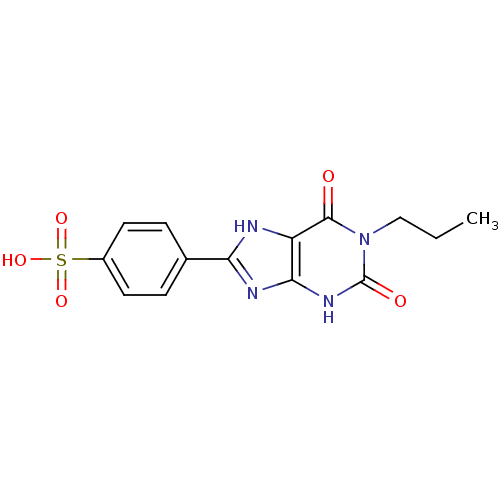 (4-(2,6-Dioxo-1-propyl-2,3,6,7-tetrahydro-1H-purin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50037429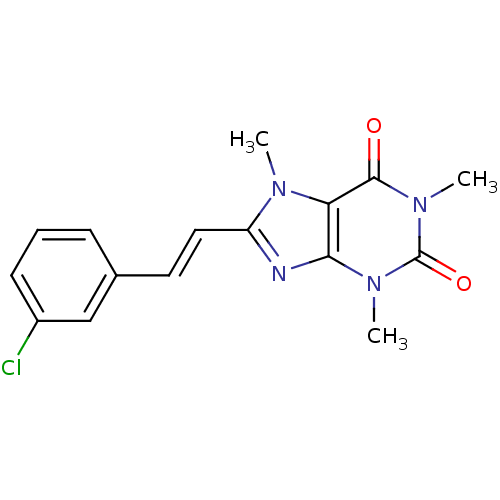 (8-(3-chlorostyryl)caffeine | 8-[(E)-2-(3-chlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118811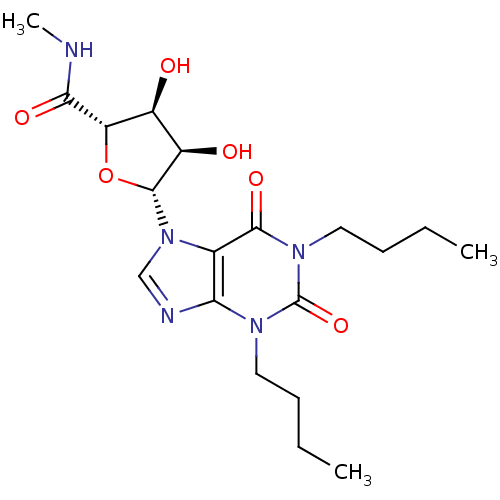 (5-(1,3-Dibutyl-2,6-dioxo-1,2,3,6-tetrahydro-purin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50018159 (4-(1,3-Dimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-pu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50391228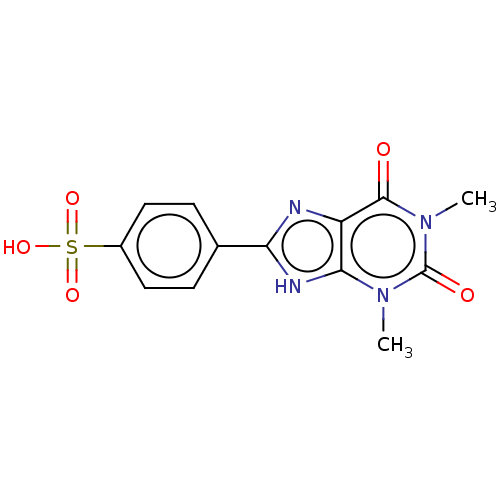 (8-(P-Sulfophenyl)Theophylline | 8-(P-Sulfophenyl)T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM50014260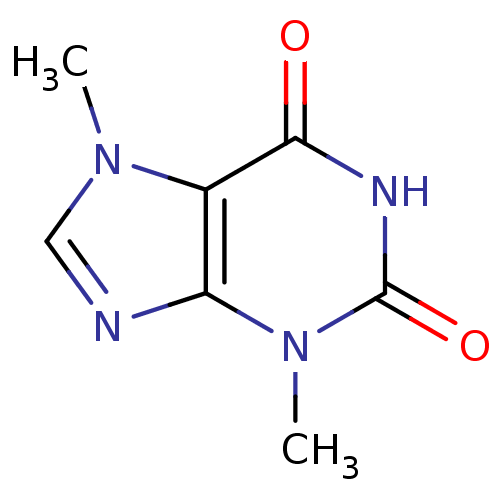 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from chicken liver | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50014260 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from chicken liver | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50014260 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Ability to inhibit U-46,619-induced contraction of isolated strips of rabbit thoracic aorta, which is the measure of thromboxane receptor antagonism | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50014260 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Ability to inhibit U-46,619-induced contraction of isolated strips of guinea pig tracheal chain, which is the measure of thromboxane receptor antagon... | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50391224 (8-PHENYL THEOPHYLLINE | 8-PT | 8-Phenyl-1,3-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50391225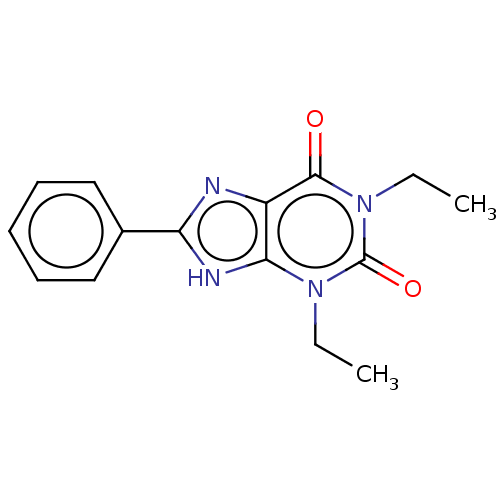 (CHEMBL11348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atria | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM10849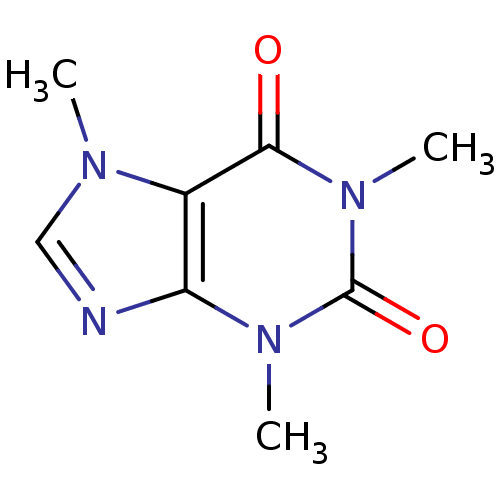 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM10849 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM10849 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM10849 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from chicken liver | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM10849 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Cardioselectivity for the beta-1 adrenergic receptor was determined against isoprenaline (antagonism) in isolated guinea pig trachea | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM50014260 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50014260 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50014260 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50014260 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from chicken liver | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50014260 (3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | 3,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coli | J Med Chem 40: 547-58 (1997) Article DOI: 10.1021/jm9604383 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514824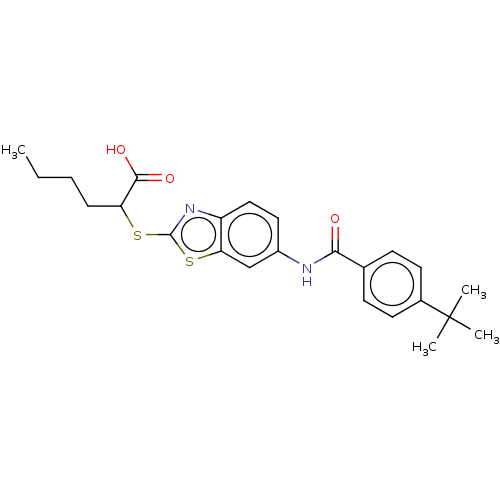 (CHEMBL4560767) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514825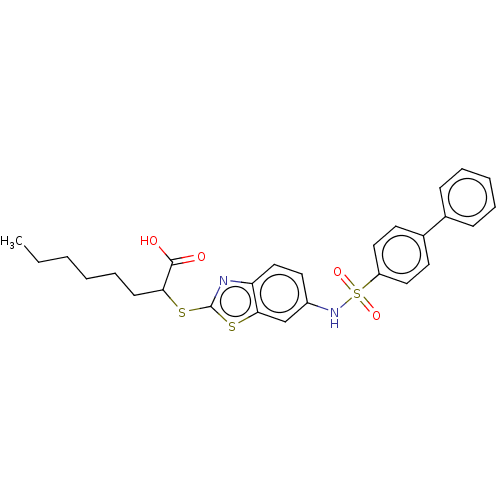 (CHEMBL4445012) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50514826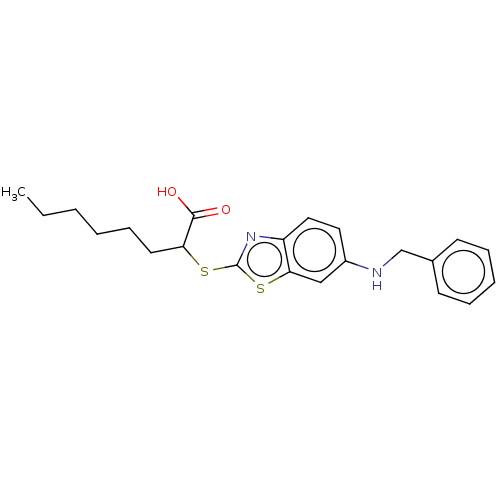 (CHEMBL4586557) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARgamma LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50514827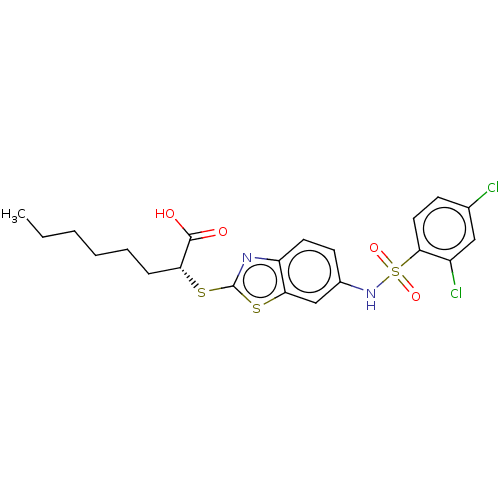 (CHEMBL4445084) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARdelta LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50514828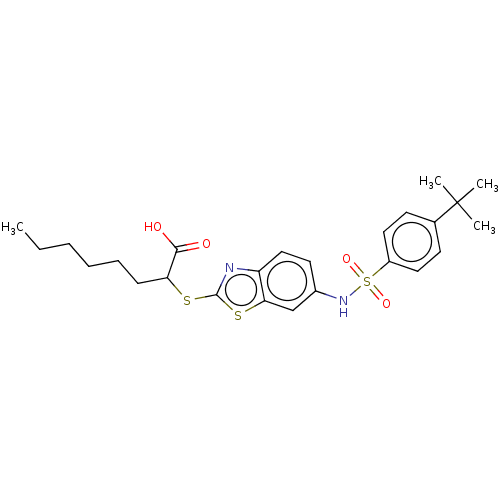 (CHEMBL4571498) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARgamma LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50514829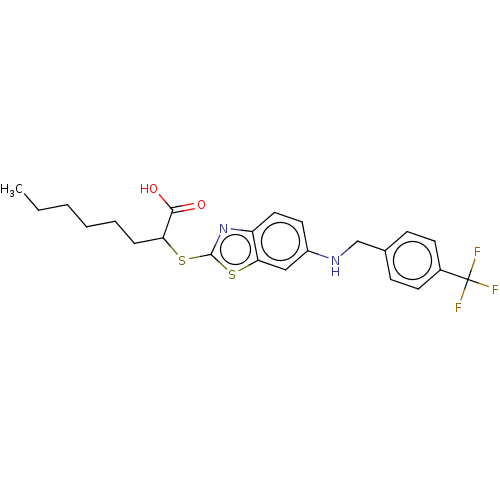 (CHEMBL4443192) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARdelta LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514830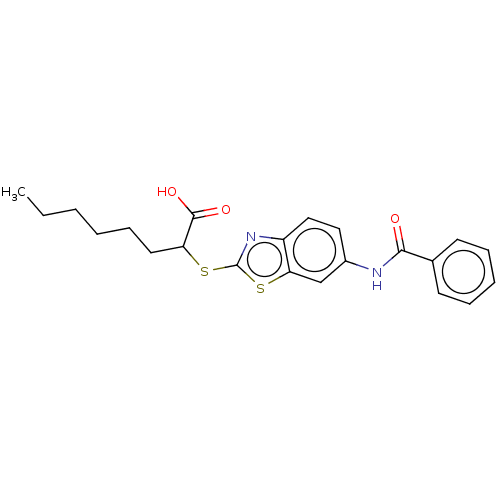 (CHEMBL4519376) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 770 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514831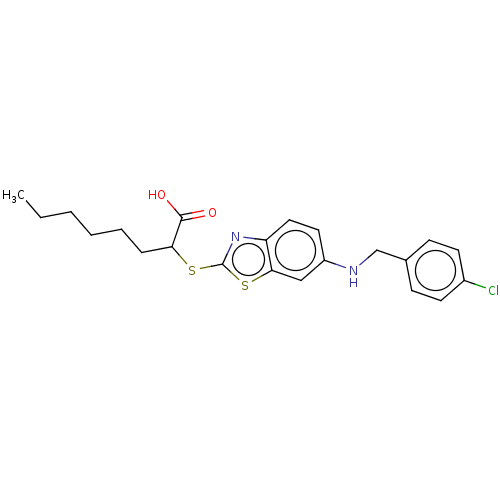 (CHEMBL4548480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514829 (CHEMBL4443192) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514832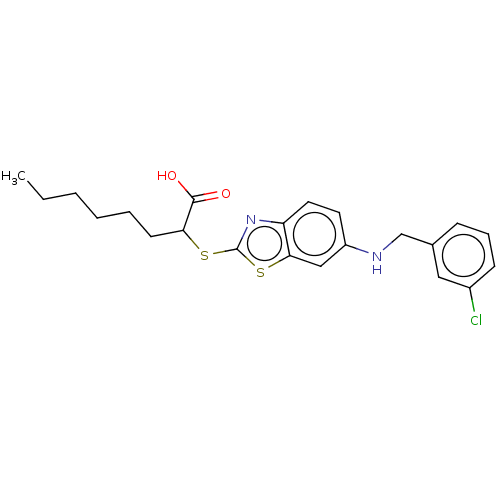 (CHEMBL4483378) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514833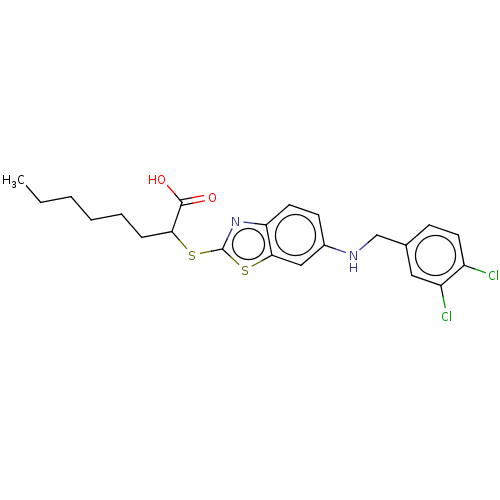 (CHEMBL4446031) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514834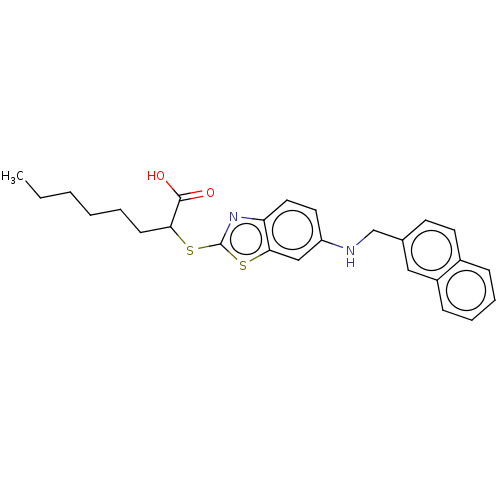 (CHEMBL4541281) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514835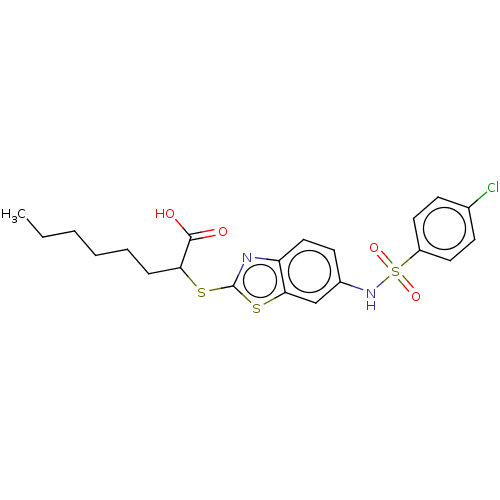 (CHEMBL4474407) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50514836 (CHEMBL4466709) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARalpha LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50514830 (CHEMBL4519376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARgamma LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50514824 (CHEMBL4560767) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARgamma LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50514837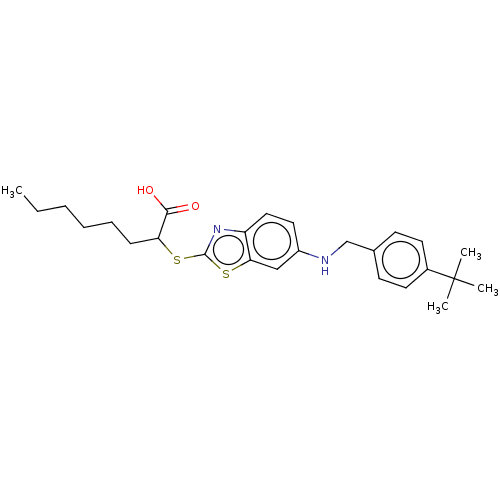 (CHEMBL4460166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at Gal4-fused PPARgamma LBD (unknown origin) expressed in HEK293T cells after 14 to 16 hrs by dual-Glo luciferase assay | J Med Chem 63: 4555-4561 (2020) Article DOI: 10.1021/acs.jmedchem.9b01786 BindingDB Entry DOI: 10.7270/Q2DB856K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 118 total ) | Next | Last >> |