 Found 90 hits with Last Name = 'feder' and Initial = 'd'
Found 90 hits with Last Name = 'feder' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fe(3+)-Zn(2+) purple acid phosphatase
(Phaseolus vulgaris) | BDBM50515728
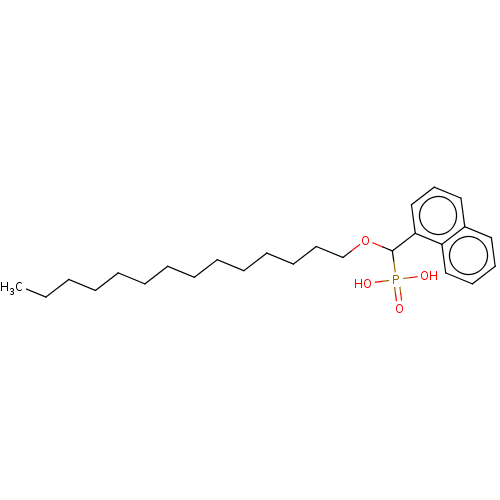
(CHEMBL4459937)Show InChI InChI=1S/C25H39O4P/c1-2-3-4-5-6-7-8-9-10-11-12-15-21-29-25(30(26,27)28)24-20-16-18-22-17-13-14-19-23(22)24/h13-14,16-20,25H,2-12,15,21H2,1H3,(H2,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean PAP using varying levels of pNPP as substrate measured at pH 6.2 by UV-vis spectrophotometric analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111611
BindingDB Entry DOI: 10.7270/Q2891960 |
More data for this
Ligand-Target Pair | |
Fe(3+)-Zn(2+) purple acid phosphatase
(Phaseolus vulgaris) | BDBM50515725

(CHEMBL4464268)Show SMILES CCCCCCCCCCCCCCCCOC(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C27H43O4P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-17-23-31-27(32(28,29)30)26-22-18-20-24-19-15-16-21-25(24)26/h15-16,18-22,27H,2-14,17,23H2,1H3,(H2,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean PAP using varying levels of pNPP as substrate measured at pH 6.2 by UV-vis spectrophotometric analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111611
BindingDB Entry DOI: 10.7270/Q2891960 |
More data for this
Ligand-Target Pair | |
Fe(3+)-Zn(2+) purple acid phosphatase
(Phaseolus vulgaris) | BDBM50515726

(CHEMBL4462405)Show SMILES CCCCCCCCCCCCCCCCCCOC(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C29H47O4P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-19-25-33-29(34(30,31)32)28-24-20-22-26-21-17-18-23-27(26)28/h17-18,20-24,29H,2-16,19,25H2,1H3,(H2,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean PAP using varying levels of pNPP as substrate measured at pH 6.2 by UV-vis spectrophotometric analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111611
BindingDB Entry DOI: 10.7270/Q2891960 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fe(3+)-Zn(2+) purple acid phosphatase
(Phaseolus vulgaris) | BDBM50515729

(CHEMBL4476405)Show SMILES CCCCCCCCCCCCCCCCCCCCOC(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C31H51O4P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-21-27-35-31(36(32,33)34)30-26-22-24-28-23-19-20-25-29(28)30/h19-20,22-26,31H,2-18,21,27H2,1H3,(H2,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean PAP using varying levels of pNPP as substrate measured at pH 6.2 by UV-vis spectrophotometric analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111611
BindingDB Entry DOI: 10.7270/Q2891960 |
More data for this
Ligand-Target Pair | |
Fe(3+)-Zn(2+) purple acid phosphatase
(Phaseolus vulgaris) | BDBM50515724
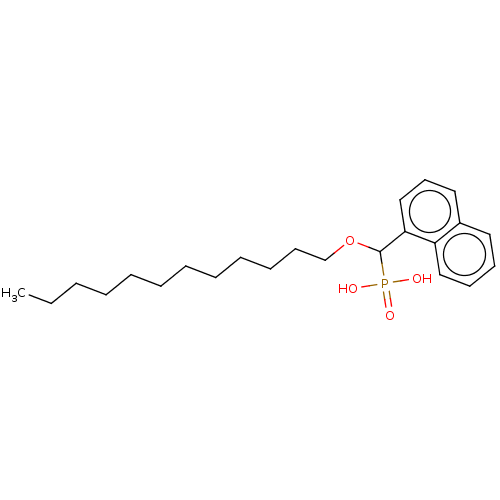
(CHEMBL4591337)Show InChI InChI=1S/C23H35O4P/c1-2-3-4-5-6-7-8-9-10-13-19-27-23(28(24,25)26)22-18-14-16-20-15-11-12-17-21(20)22/h11-12,14-18,23H,2-10,13,19H2,1H3,(H2,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean PAP using varying levels of pNPP as substrate measured at pH 6.2 by UV-vis spectrophotometric analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111611
BindingDB Entry DOI: 10.7270/Q2891960 |
More data for this
Ligand-Target Pair | |
Fe(3+)-Zn(2+) purple acid phosphatase
(Phaseolus vulgaris) | BDBM50515727
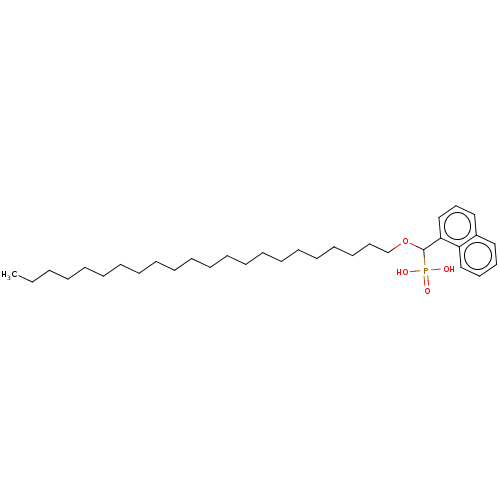
(CHEMBL4442978)Show SMILES CCCCCCCCCCCCCCCCCCCCCCOC(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C33H55O4P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-23-29-37-33(38(34,35)36)32-28-24-26-30-25-21-22-27-31(30)32/h21-22,24-28,33H,2-20,23,29H2,1H3,(H2,34,35,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean PAP using varying levels of pNPP as substrate measured at pH 6.2 by UV-vis spectrophotometric analysis |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111611
BindingDB Entry DOI: 10.7270/Q2891960 |
More data for this
Ligand-Target Pair | |
Tartrate-resistant acid phosphatase type 5
(Sus scrofa) | BDBM50469090
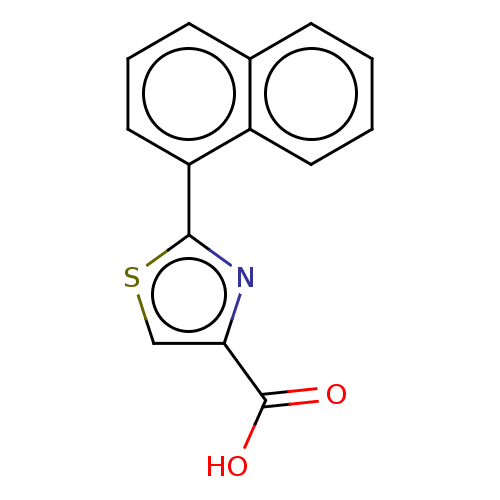
(CHEMBL4288392)Show InChI InChI=1S/C14H9NO2S/c16-14(17)12-8-18-13(15-12)11-7-3-5-9-4-1-2-6-10(9)11/h1-8H,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV/Vis spectro... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Tartrate-resistant acid phosphatase type 5
(Sus scrofa) | BDBM50469089
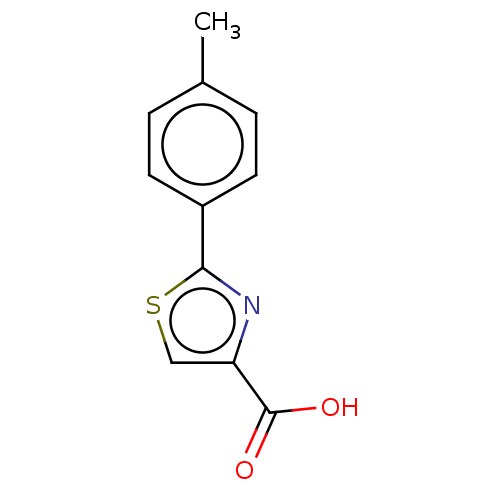
(CHEMBL1405779)Show InChI InChI=1S/C11H9NO2S/c1-7-2-4-8(5-3-7)10-12-9(6-15-10)11(13)14/h2-6H,1H3,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV/Vis spectro... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Tartrate-resistant acid phosphatase type 5
(Sus scrofa) | BDBM55840
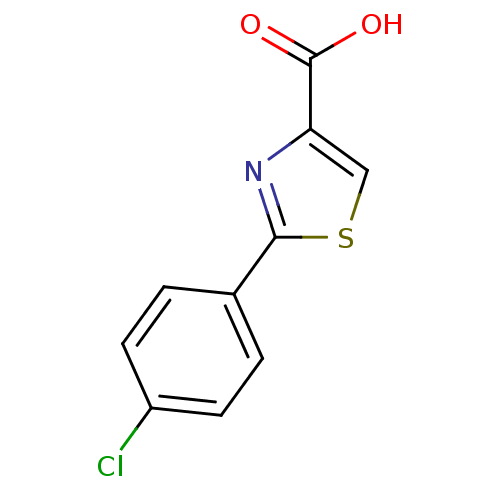
(2-(4-chlorophenyl)-1,3-thiazole-4-carboxylic acid ...)Show InChI InChI=1S/C10H6ClNO2S/c11-7-3-1-6(2-4-7)9-12-8(5-15-9)10(13)14/h1-5H,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV/Vis spectro... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Tartrate-resistant acid phosphatase type 5
(Sus scrofa) | BDBM50469088
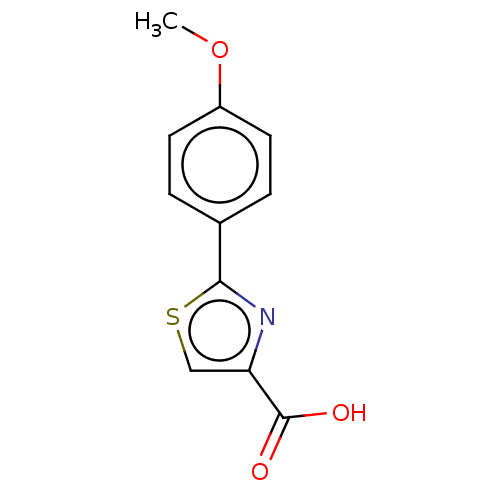
(CHEMBL4287988)Show InChI InChI=1S/C11H9NO3S/c1-15-8-4-2-7(3-5-8)10-12-9(6-16-10)11(13)14/h2-6H,1H3,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV/Vis spectro... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Fe(3+)-Zn(2+) purple acid phosphatase
(Phaseolus vulgaris) | BDBM50311531

((2-Carboxyethyl)-phosphonic acid | 3-PHOSPHONOPROP...)Show InChI InChI=1S/C3H7O5P/c4-3(5)1-2-9(6,7)8/h1-2H2,(H,4,5)(H2,6,7,8) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of red kidney bean PAP using pNPP as substrate |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111611
BindingDB Entry DOI: 10.7270/Q2891960 |
More data for this
Ligand-Target Pair | |
Purple acid phosphatase
(Phaseolus vulgaris) | BDBM50469090

(CHEMBL4288392)Show InChI InChI=1S/C14H9NO2S/c16-14(17)12-8-18-13(15-12)11-7-3-5-9-4-1-2-6-10(9)11/h1-8H,(H,16,17) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Tartrate-resistant acid phosphatase type 5
(Sus scrofa) | BDBM63710

(2-phenyl-1,3-thiazole-4-carboxylic acid | 2-phenyl...)Show InChI InChI=1S/C10H7NO2S/c12-10(13)8-6-14-9(11-8)7-4-2-1-3-5-7/h1-6H,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV/Vis spectro... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Purple acid phosphatase
(Phaseolus vulgaris) | BDBM50469089

(CHEMBL1405779)Show InChI InChI=1S/C11H9NO2S/c1-7-2-4-8(5-3-7)10-12-9(6-15-10)11(13)14/h2-6H,1H3,(H,13,14) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Purple acid phosphatase
(Phaseolus vulgaris) | BDBM50469088

(CHEMBL4287988)Show InChI InChI=1S/C11H9NO3S/c1-15-8-4-2-7(3-5-8)10-12-9(6-16-10)11(13)14/h2-6H,1H3,(H,13,14) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Purple acid phosphatase
(Phaseolus vulgaris) | BDBM55840

(2-(4-chlorophenyl)-1,3-thiazole-4-carboxylic acid ...)Show InChI InChI=1S/C10H6ClNO2S/c11-7-3-1-6(2-4-7)9-12-8(5-15-9)10(13)14/h1-5H,(H,13,14) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Tartrate-resistant acid phosphatase type 5
(Sus scrofa) | BDBM50469091
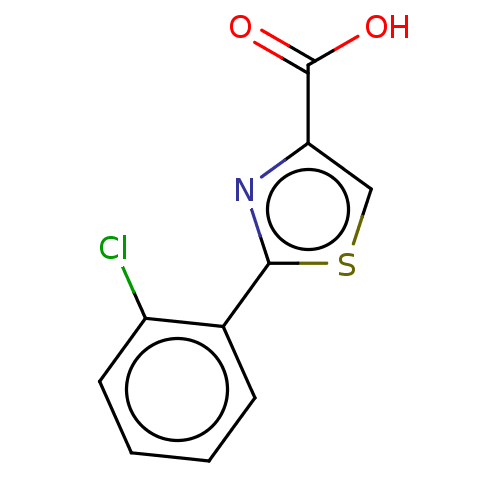
(CHEMBL4283261)Show InChI InChI=1S/C10H6ClNO2S/c11-7-4-2-1-3-6(7)9-12-8(5-15-9)10(13)14/h1-5H,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV/Vis spectro... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Purple acid phosphatase
(Phaseolus vulgaris) | BDBM50469091

(CHEMBL4283261)Show InChI InChI=1S/C10H6ClNO2S/c11-7-4-2-1-3-6(7)9-12-8(5-15-9)10(13)14/h1-5H,(H,13,14) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 9.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Competitive inhibition of red kidney bean purple acid phosphatase Fe(3)Fe(2) state assessed as enzyme-inhibitor complex using pNPP as substrate by UV... |
Eur J Med Chem 157: 462-479 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KNV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50421295
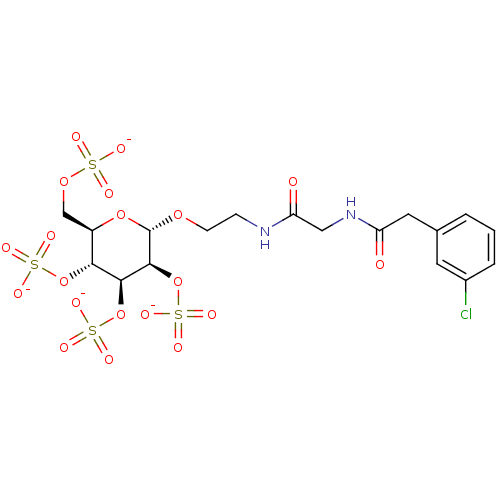
(CHEMBL2087907)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCNC(=O)CNC(=O)Cc2cccc(Cl)c2)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C18H25ClN2O20S4/c19-11-3-1-2-10(6-11)7-13(22)21-8-14(23)20-4-5-36-18-17(41-45(33,34)35)16(40-44(30,31)32)15(39-43(27,28)29)12(38-18)9-37-42(24,25)26/h1-3,6,12,15-18H,4-5,7-9H2,(H,20,23)(H,21,22)(H,24,25,26)(H,27,28,29)(H,30,31,32)(H,33,34,35)/p-4/t12-,15-,16+,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to VEGF by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50421296
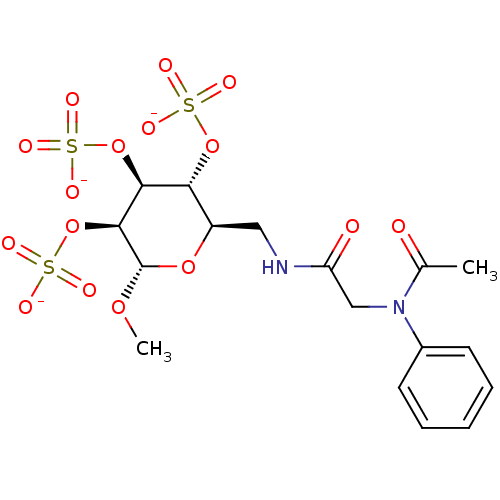
(CHEMBL2087888)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(C(C)=O)c2ccccc2)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C17H24N2O16S3/c1-10(20)19(11-6-4-3-5-7-11)9-13(21)18-8-12-14(33-36(22,23)24)15(34-37(25,26)27)16(17(31-2)32-12)35-38(28,29)30/h3-7,12,14-17H,8-9H2,1-2H3,(H,18,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)/p-3/t12-,14-,15+,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to VEGF by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421297

(CHEMBL2087893)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(C2CCCCCCCCCCC2)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C23H42N2O16S3/c1-16(26)25(17-12-10-8-6-4-3-5-7-9-11-13-17)15-19(27)24-14-18-20(39-42(28,29)30)21(40-43(31,32)33)22(23(37-2)38-18)41-44(34,35)36/h17-18,20-23H,3-15H2,1-2H3,(H,24,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)/p-3/t18-,20-,21+,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421298

(CHEMBL2087889)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(Cc2ccccc2)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C18H26N2O16S3/c1-11(21)20(9-12-6-4-3-5-7-12)10-14(22)19-8-13-15(34-37(23,24)25)16(35-38(26,27)28)17(18(32-2)33-13)36-39(29,30)31/h3-7,13,15-18H,8-10H2,1-2H3,(H,19,22)(H,23,24,25)(H,26,27,28)(H,29,30,31)/p-3/t13-,15-,16+,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421299
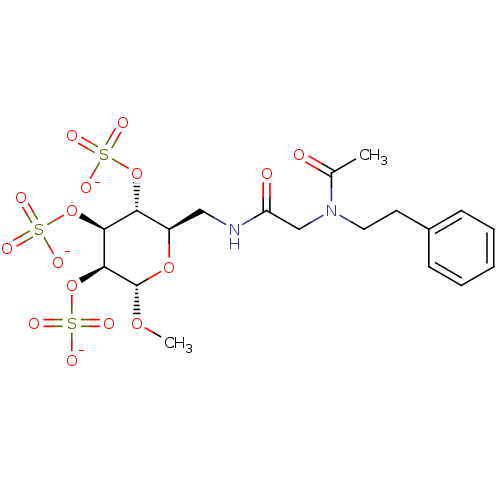
(CHEMBL2087890)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(CCc2ccccc2)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C19H28N2O16S3/c1-12(22)21(9-8-13-6-4-3-5-7-13)11-15(23)20-10-14-16(35-38(24,25)26)17(36-39(27,28)29)18(19(33-2)34-14)37-40(30,31)32/h3-7,14,16-19H,8-11H2,1-2H3,(H,20,23)(H,24,25,26)(H,27,28,29)(H,30,31,32)/p-3/t14-,16-,17+,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.24E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421300
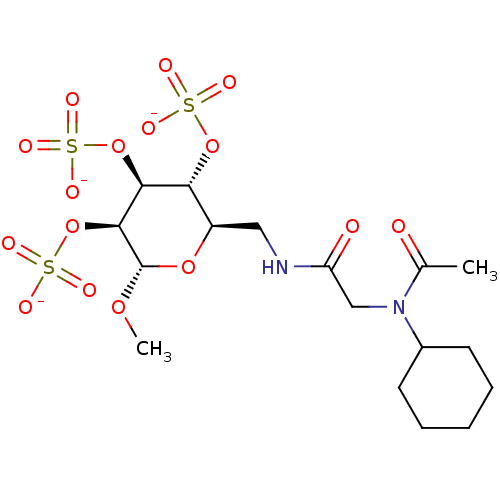
(CHEMBL2087891)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(C2CCCCC2)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C17H30N2O16S3/c1-10(20)19(11-6-4-3-5-7-11)9-13(21)18-8-12-14(33-36(22,23)24)15(34-37(25,26)27)16(17(31-2)32-12)35-38(28,29)30/h11-12,14-17H,3-9H2,1-2H3,(H,18,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)/p-3/t12-,14-,15+,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.10E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421297

(CHEMBL2087893)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(C2CCCCCCCCCCC2)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C23H42N2O16S3/c1-16(26)25(17-12-10-8-6-4-3-5-7-9-11-13-17)15-19(27)24-14-18-20(39-42(28,29)30)21(40-43(31,32)33)22(23(37-2)38-18)41-44(34,35)36/h17-18,20-23H,3-15H2,1-2H3,(H,24,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)/p-3/t18-,20-,21+,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.65E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421301

(CHEMBL2087894)Show SMILES CCCCC1CCC(CC1)N(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(C)=O |r,wU:16.16,27.28,21.22,wD:33.35,18.19,(14.12,3.67,;12.79,2.9,;12.78,1.36,;11.45,.59,;11.45,-.95,;10.12,-1.73,;10.12,-3.27,;11.45,-4.03,;12.78,-3.27,;12.77,-1.73,;11.45,-5.57,;10.12,-6.33,;8.78,-5.56,;8.79,-4.02,;7.45,-6.32,;6.12,-5.55,;4.78,-6.32,;4.78,-7.86,;3.45,-8.62,;3.45,-10.16,;4.79,-10.93,;2.12,-7.86,;.79,-8.63,;.79,-10.17,;-.54,-10.94,;2.33,-10.17,;1.55,-11.5,;2.12,-6.32,;.79,-5.55,;-.54,-6.32,;-1.88,-5.56,;-1.32,-7.65,;.22,-7.65,;3.45,-5.54,;3.45,-4,;2.12,-3.23,;.78,-4,;1.34,-1.89,;2.88,-1.89,;12.78,-6.34,;12.78,-7.88,;14.12,-5.57,)| Show InChI InChI=1S/C21H38N2O16S3/c1-4-5-6-14-7-9-15(10-8-14)23(13(2)24)12-17(25)22-11-16-18(37-40(26,27)28)19(38-41(29,30)31)20(21(35-3)36-16)39-42(32,33)34/h14-16,18-21H,4-12H2,1-3H3,(H,22,25)(H,26,27,28)(H,29,30,31)(H,32,33,34)/p-3/t14?,15?,16-,18-,19+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.43E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421302
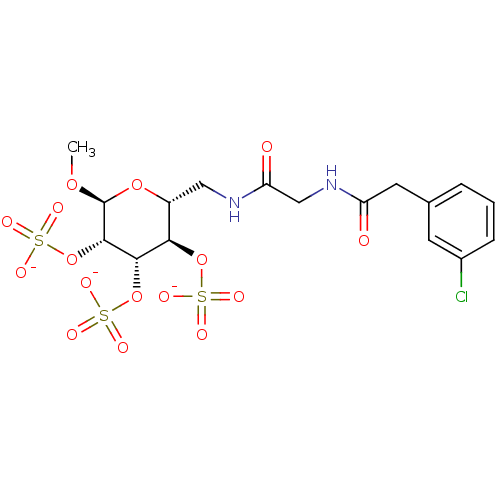
(CHEMBL2087895)Show SMILES CO[C@H]1O[C@H](CNC(=O)CNC(=O)Cc2cccc(Cl)c2)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C17H23ClN2O16S3/c1-32-17-16(36-39(29,30)31)15(35-38(26,27)28)14(34-37(23,24)25)11(33-17)7-19-13(22)8-20-12(21)6-9-3-2-4-10(18)5-9/h2-5,11,14-17H,6-8H2,1H3,(H,19,22)(H,20,21)(H,23,24,25)(H,26,27,28)(H,29,30,31)/p-3/t11-,14-,15+,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421303
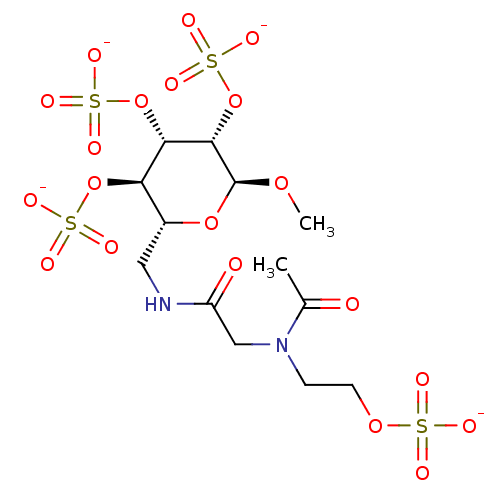
(CHEMBL2087896)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(CCOS([O-])(=O)=O)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C13H24N2O20S4/c1-7(16)15(3-4-31-36(18,19)20)6-9(17)14-5-8-10(33-37(21,22)23)11(34-38(24,25)26)12(13(30-2)32-8)35-39(27,28)29/h8,10-13H,3-6H2,1-2H3,(H,14,17)(H,18,19,20)(H,21,22,23)(H,24,25,26)(H,27,28,29)/p-4/t8-,10-,11+,12+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421304
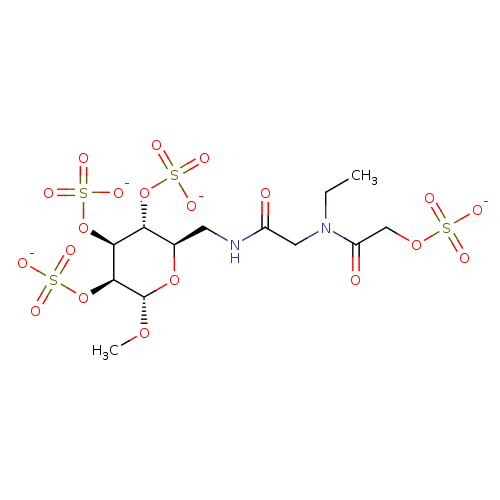
(CHEMBL2087897)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)COS([O-])(=O)=O |r| Show InChI InChI=1S/C13H24N2O20S4/c1-3-15(9(17)6-31-36(18,19)20)5-8(16)14-4-7-10(33-37(21,22)23)11(34-38(24,25)26)12(13(30-2)32-7)35-39(27,28)29/h7,10-13H,3-6H2,1-2H3,(H,14,16)(H,18,19,20)(H,21,22,23)(H,24,25,26)(H,27,28,29)/p-4/t7-,10-,11+,12+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.74E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421305
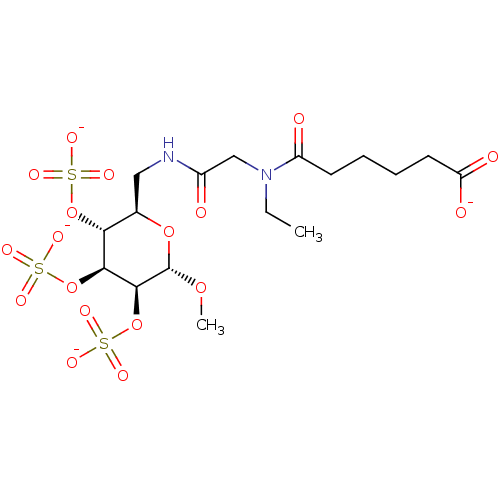
(CHEMBL2087899)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)CCCCC([O-])=O |r| Show InChI InChI=1S/C17H30N2O18S3/c1-3-19(12(21)6-4-5-7-13(22)23)9-11(20)18-8-10-14(35-38(24,25)26)15(36-39(27,28)29)16(17(33-2)34-10)37-40(30,31)32/h10,14-17H,3-9H2,1-2H3,(H,18,20)(H,22,23)(H,24,25,26)(H,27,28,29)(H,30,31,32)/p-4/t10-,14-,15+,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421306
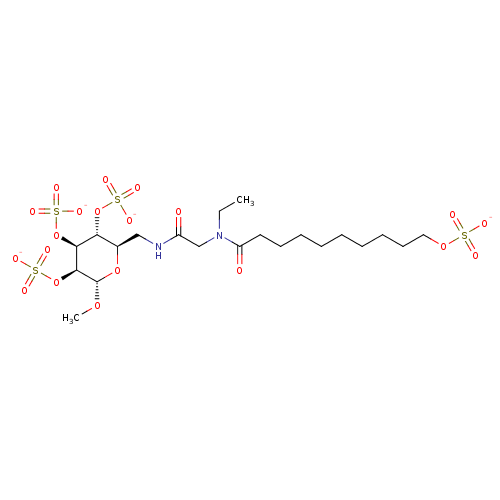
(CHEMBL2087900)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)CCCCCCCCCOS([O-])(=O)=O |r| Show InChI InChI=1S/C21H40N2O20S4/c1-3-23(17(25)11-9-7-5-4-6-8-10-12-39-44(26,27)28)14-16(24)22-13-15-18(41-45(29,30)31)19(42-46(32,33)34)20(21(38-2)40-15)43-47(35,36)37/h15,18-21H,3-14H2,1-2H3,(H,22,24)(H,26,27,28)(H,29,30,31)(H,32,33,34)(H,35,36,37)/p-4/t15-,18-,19+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.11E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421307
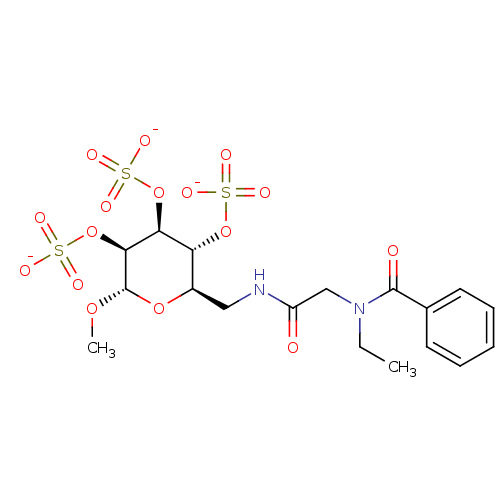
(CHEMBL2087901)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C18H26N2O16S3/c1-3-20(17(22)11-7-5-4-6-8-11)10-13(21)19-9-12-14(34-37(23,24)25)15(35-38(26,27)28)16(18(32-2)33-12)36-39(29,30)31/h4-8,12,14-16,18H,3,9-10H2,1-2H3,(H,19,21)(H,23,24,25)(H,26,27,28)(H,29,30,31)/p-3/t12-,14-,15+,16+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.66E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421308

(CHEMBL2087903)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)CCCc1ccccc1 |r| Show InChI InChI=1S/C21H32N2O16S3/c1-3-23(17(25)11-7-10-14-8-5-4-6-9-14)13-16(24)22-12-15-18(37-40(26,27)28)19(38-41(29,30)31)20(21(35-2)36-15)39-42(32,33)34/h4-6,8-9,15,18-21H,3,7,10-13H2,1-2H3,(H,22,24)(H,26,27,28)(H,29,30,31)(H,32,33,34)/p-3/t15-,18-,19+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421309
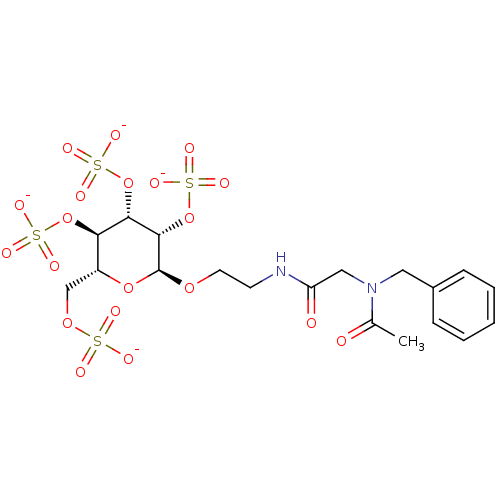
(CHEMBL2087905)Show SMILES CC(=O)N(CC(=O)NCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C19H28N2O20S4/c1-12(22)21(9-13-5-3-2-4-6-13)10-15(23)20-7-8-36-19-18(41-45(33,34)35)17(40-44(30,31)32)16(39-43(27,28)29)14(38-19)11-37-42(24,25)26/h2-6,14,16-19H,7-11H2,1H3,(H,20,23)(H,24,25,26)(H,27,28,29)(H,30,31,32)(H,33,34,35)/p-4/t14-,16-,17+,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421310
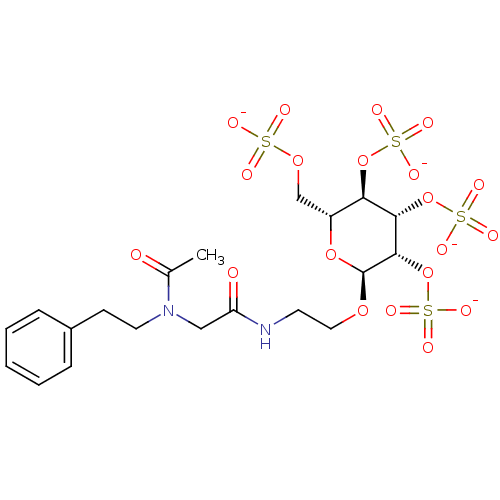
(CHEMBL2087906)Show SMILES CC(=O)N(CCc1ccccc1)CC(=O)NCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C20H30N2O20S4/c1-13(23)22(9-7-14-5-3-2-4-6-14)11-16(24)21-8-10-37-20-19(42-46(34,35)36)18(41-45(31,32)33)17(40-44(28,29)30)15(39-20)12-38-43(25,26)27/h2-6,15,17-20H,7-12H2,1H3,(H,21,24)(H,25,26,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)/p-4/t15-,17-,18+,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421311

(CHEMBL2087908)Show SMILES CCN(CC(=O)NCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)CCC([O-])=O |r| Show InChI InChI=1S/C16H28N2O22S4/c1-2-18(11(20)3-4-12(21)22)7-10(19)17-5-6-35-16-15(40-44(32,33)34)14(39-43(29,30)31)13(38-42(26,27)28)9(37-16)8-36-41(23,24)25/h9,13-16H,2-8H2,1H3,(H,17,19)(H,21,22)(H,23,24,25)(H,26,27,28)(H,29,30,31)(H,32,33,34)/p-5/t9-,13-,14+,15+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421312
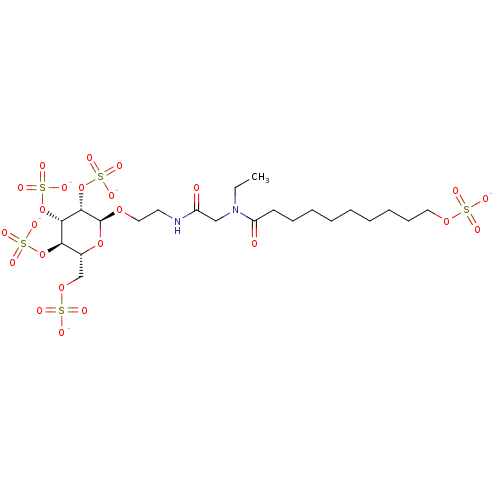
(CHEMBL2087910)Show SMILES CCN(CC(=O)NCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)CCCCCCCCCOS([O-])(=O)=O |r| Show InChI InChI=1S/C22H42N2O24S5/c1-2-24(18(26)10-8-6-4-3-5-7-9-12-43-49(27,28)29)14-17(25)23-11-13-42-22-21(48-53(39,40)41)20(47-52(36,37)38)19(46-51(33,34)35)16(45-22)15-44-50(30,31)32/h16,19-22H,2-15H2,1H3,(H,23,25)(H,27,28,29)(H,30,31,32)(H,33,34,35)(H,36,37,38)(H,39,40,41)/p-5/t16-,19-,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421313
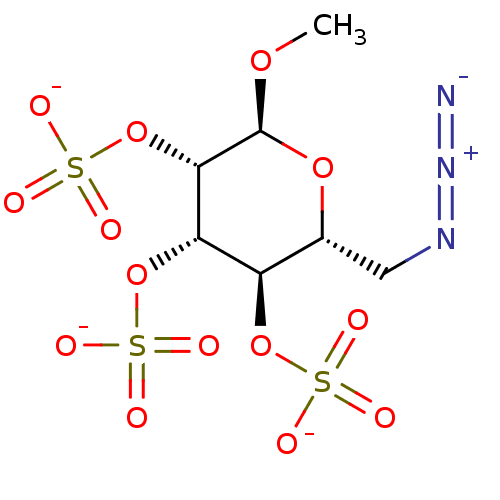
(CHEMBL2087911)Show SMILES CO[C@H]1O[C@H](CN=[N+]=[N-])[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C7H13N3O14S3/c1-20-7-6(24-27(17,18)19)5(23-26(14,15)16)4(22-25(11,12)13)3(21-7)2-9-10-8/h3-7H,2H2,1H3,(H,11,12,13)(H,14,15,16)(H,17,18,19)/p-3/t3-,4-,5+,6+,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.43E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50421314
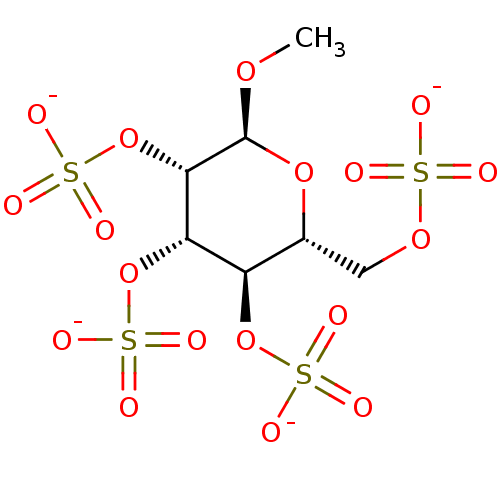
(CHEMBL2087912)Show SMILES CO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C7H14O18S4/c1-20-7-6(25-29(17,18)19)5(24-28(14,15)16)4(23-27(11,12)13)3(22-7)2-21-26(8,9)10/h3-7H,2H2,1H3,(H,8,9,10)(H,11,12,13)(H,14,15,16)(H,17,18,19)/p-4/t3-,4-,5+,6+,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421296

(CHEMBL2087888)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(C(C)=O)c2ccccc2)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C17H24N2O16S3/c1-10(20)19(11-6-4-3-5-7-11)9-13(21)18-8-12-14(33-36(22,23)24)15(34-37(25,26)27)16(17(31-2)32-12)35-38(28,29)30/h3-7,12,14-17H,8-9H2,1-2H3,(H,18,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)/p-3/t12-,14-,15+,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421299

(CHEMBL2087890)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(CCc2ccccc2)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C19H28N2O16S3/c1-12(22)21(9-8-13-6-4-3-5-7-13)11-15(23)20-10-14-16(35-38(24,25)26)17(36-39(27,28)29)18(19(33-2)34-14)37-40(30,31)32/h3-7,14,16-19H,8-11H2,1-2H3,(H,20,23)(H,24,25,26)(H,27,28,29)(H,30,31,32)/p-3/t14-,16-,17+,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.80E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421300

(CHEMBL2087891)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(C2CCCCC2)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C17H30N2O16S3/c1-10(20)19(11-6-4-3-5-7-11)9-13(21)18-8-12-14(33-36(22,23)24)15(34-37(25,26)27)16(17(31-2)32-12)35-38(28,29)30/h11-12,14-17H,3-9H2,1-2H3,(H,18,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)/p-3/t12-,14-,15+,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.13E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421315
(CHEMBL2087892)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(C2CCCCCC2)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C18H32N2O16S3/c1-11(21)20(12-7-5-3-4-6-8-12)10-14(22)19-9-13-15(34-37(23,24)25)16(35-38(26,27)28)17(18(32-2)33-13)36-39(29,30)31/h12-13,15-18H,3-10H2,1-2H3,(H,19,22)(H,23,24,25)(H,26,27,28)(H,29,30,31)/p-3/t13-,15-,16+,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.10E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421301

(CHEMBL2087894)Show SMILES CCCCC1CCC(CC1)N(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(C)=O |r,wU:16.16,27.28,21.22,wD:33.35,18.19,(14.12,3.67,;12.79,2.9,;12.78,1.36,;11.45,.59,;11.45,-.95,;10.12,-1.73,;10.12,-3.27,;11.45,-4.03,;12.78,-3.27,;12.77,-1.73,;11.45,-5.57,;10.12,-6.33,;8.78,-5.56,;8.79,-4.02,;7.45,-6.32,;6.12,-5.55,;4.78,-6.32,;4.78,-7.86,;3.45,-8.62,;3.45,-10.16,;4.79,-10.93,;2.12,-7.86,;.79,-8.63,;.79,-10.17,;-.54,-10.94,;2.33,-10.17,;1.55,-11.5,;2.12,-6.32,;.79,-5.55,;-.54,-6.32,;-1.88,-5.56,;-1.32,-7.65,;.22,-7.65,;3.45,-5.54,;3.45,-4,;2.12,-3.23,;.78,-4,;1.34,-1.89,;2.88,-1.89,;12.78,-6.34,;12.78,-7.88,;14.12,-5.57,)| Show InChI InChI=1S/C21H38N2O16S3/c1-4-5-6-14-7-9-15(10-8-14)23(13(2)24)12-17(25)22-11-16-18(37-40(26,27)28)19(38-41(29,30)31)20(21(35-3)36-16)39-42(32,33)34/h14-16,18-21H,4-12H2,1-3H3,(H,22,25)(H,26,27,28)(H,29,30,31)(H,32,33,34)/p-3/t14?,15?,16-,18-,19+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.40E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421303

(CHEMBL2087896)Show SMILES CO[C@H]1O[C@H](CNC(=O)CN(CCOS([O-])(=O)=O)C(C)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C13H24N2O20S4/c1-7(16)15(3-4-31-36(18,19)20)6-9(17)14-5-8-10(33-37(21,22)23)11(34-38(24,25)26)12(13(30-2)32-8)35-39(27,28)29/h8,10-13H,3-6H2,1-2H3,(H,14,17)(H,18,19,20)(H,21,22,23)(H,24,25,26)(H,27,28,29)/p-4/t8-,10-,11+,12+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
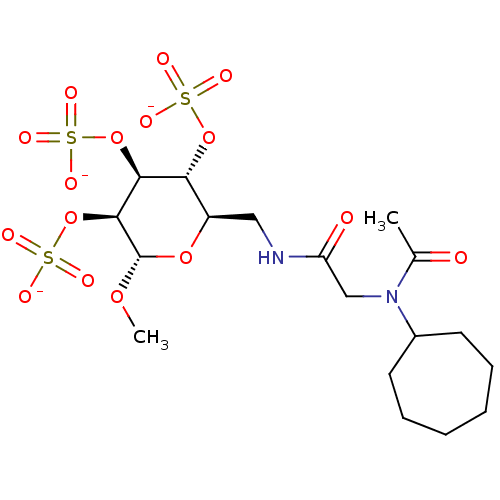
(Homo sapiens (Human)) | BDBM50421316
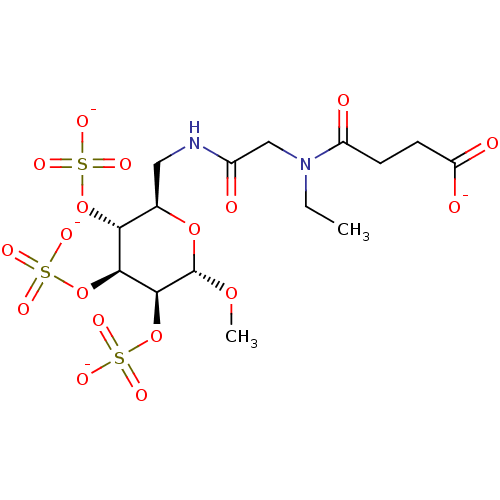
(CHEMBL2087898)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)CCC([O-])=O |r| Show InChI InChI=1S/C15H26N2O18S3/c1-3-17(10(19)4-5-11(20)21)7-9(18)16-6-8-12(33-36(22,23)24)13(34-37(25,26)27)14(15(31-2)32-8)35-38(28,29)30/h8,12-15H,3-7H2,1-2H3,(H,16,18)(H,20,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)/p-4/t8-,12-,13+,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.91E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421305

(CHEMBL2087899)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)CCCCC([O-])=O |r| Show InChI InChI=1S/C17H30N2O18S3/c1-3-19(12(21)6-4-5-7-13(22)23)9-11(20)18-8-10-14(35-38(24,25)26)15(36-39(27,28)29)16(17(33-2)34-10)37-40(30,31)32/h10,14-17H,3-9H2,1-2H3,(H,18,20)(H,22,23)(H,24,25,26)(H,27,28,29)(H,30,31,32)/p-4/t10-,14-,15+,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421307

(CHEMBL2087901)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C18H26N2O16S3/c1-3-20(17(22)11-7-5-4-6-8-11)10-13(21)19-9-12-14(34-37(23,24)25)15(35-38(26,27)28)16(18(32-2)33-12)36-39(29,30)31/h4-8,12,14-16,18H,3,9-10H2,1-2H3,(H,19,21)(H,23,24,25)(H,26,27,28)(H,29,30,31)/p-3/t12-,14-,15+,16+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421308

(CHEMBL2087903)Show SMILES CCN(CC(=O)NC[C@H]1O[C@H](OC)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)C(=O)CCCc1ccccc1 |r| Show InChI InChI=1S/C21H32N2O16S3/c1-3-23(17(25)11-7-10-14-8-5-4-6-9-14)13-16(24)22-12-15-18(37-40(26,27)28)19(38-41(29,30)31)20(21(35-2)36-15)39-42(32,33)34/h4-6,8-9,15,18-21H,3,7,10-13H2,1-2H3,(H,22,24)(H,26,27,28)(H,29,30,31)(H,32,33,34)/p-3/t15-,18-,19+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 2
(Homo sapiens (Human)) | BDBM50421317
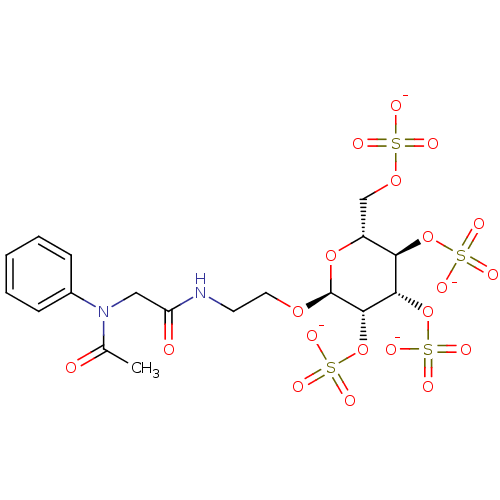
(CHEMBL2087904)Show SMILES CC(=O)N(CC(=O)NCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C18H26N2O20S4/c1-11(21)20(12-5-3-2-4-6-12)9-14(22)19-7-8-35-18-17(40-44(32,33)34)16(39-43(29,30)31)15(38-42(26,27)28)13(37-18)10-36-41(23,24)25/h2-6,13,15-18H,7-10H2,1H3,(H,19,22)(H,23,24,25)(H,26,27,28)(H,29,30,31)(H,32,33,34)/p-4/t13-,15-,16+,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity to FGF2 by surface plasmon resonance-based solution affinity assay |
Bioorg Med Chem Lett 22: 6190-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.001
BindingDB Entry DOI: 10.7270/Q2QR4ZD6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data