 Found 21 hits with Last Name = 'fensholdt' and Initial = 'j'
Found 21 hits with Last Name = 'fensholdt' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50416875
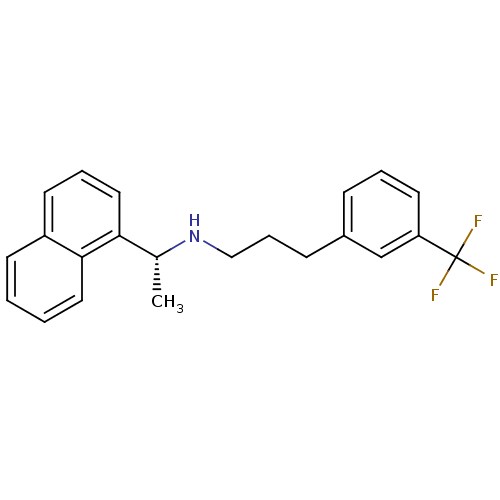
(AMG-073 | AMG073 HCL | CINACALCET | CINACALCET HYD...)Show SMILES C[C@@H](NCCCc1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C22H22F3N/c1-16(20-13-5-10-18-9-2-3-12-21(18)20)26-14-6-8-17-7-4-11-19(15-17)22(23,24)25/h2-5,7,9-13,15-16,26H,6,8,14H2,1H3/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4851
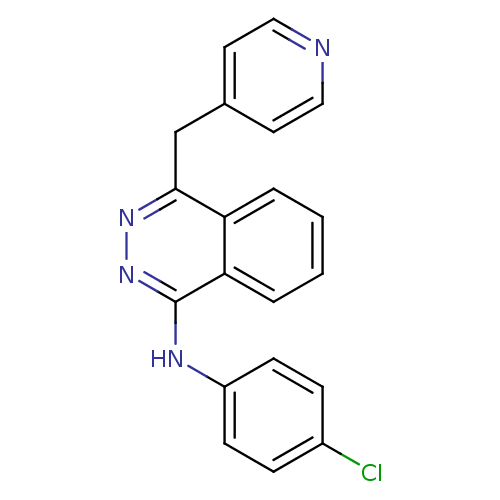
((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...)Show InChI InChI=1S/C20H15ClN4/c21-15-5-7-16(8-6-15)23-20-18-4-2-1-3-17(18)19(24-25-20)13-14-9-11-22-12-10-14/h1-12H,13H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM17028
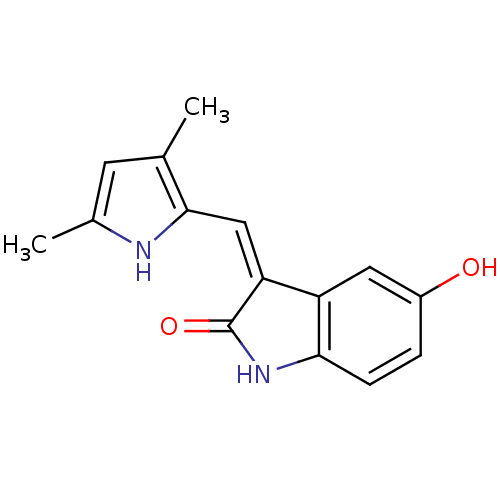
((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...)Show InChI InChI=1S/C15H14N2O2/c1-8-5-9(2)16-14(8)7-12-11-6-10(18)3-4-13(11)17-15(12)19/h3-7,16,18H,1-2H3,(H,17,19)/b12-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM17015

((3Z)-3-(1H-pyrrol-2-ylmethylidene)-2,3-dihydro-1H-...)Show InChI InChI=1S/C13H10N2O/c16-13-11(8-9-4-3-7-14-9)10-5-1-2-6-12(10)15-13/h1-8,14H,(H,15,16)/b11-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4810
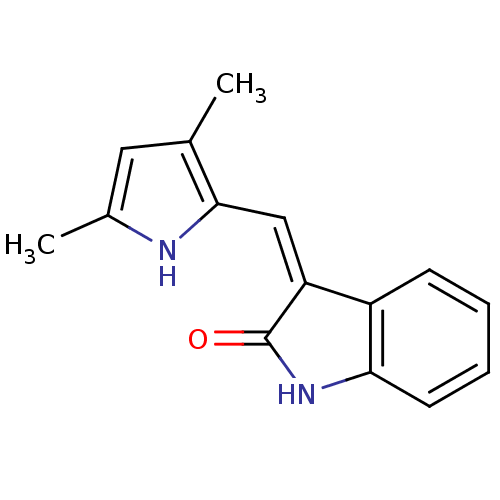
((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...)Show InChI InChI=1S/C15H14N2O/c1-9-7-10(2)16-14(9)8-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50171851

(3-[1-(4-Bromo-3,5-dimethyl-1H-pyrrol-2-yl)-meth-(Z...)Show InChI InChI=1S/C15H13BrN2O/c1-8-13(17-9(2)14(8)16)7-11-10-5-3-4-6-12(10)18-15(11)19/h3-7,17H,1-2H3,(H,18,19)/b11-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50171852
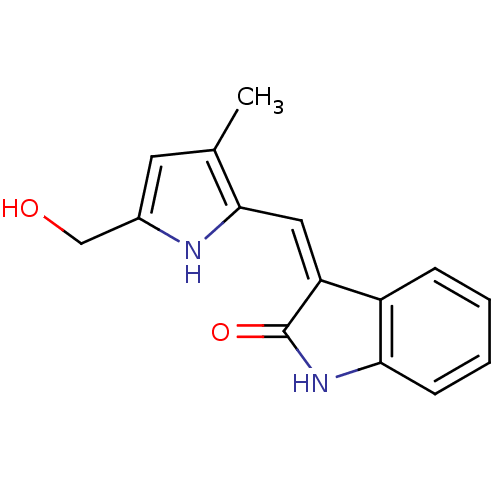
(3-[1-(5-Hydroxymethyl-3-methyl-1H-pyrrol-2-yl)-met...)Show InChI InChI=1S/C15H14N2O2/c1-9-6-10(8-18)16-14(9)7-12-11-4-2-3-5-13(11)17-15(12)19/h2-7,16,18H,8H2,1H3,(H,17,19)/b12-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50171858

(Acetic acid 3-[1-(3,5-dimethyl-1H-pyrrol-2-yl)-met...)Show SMILES CC(=O)ON1C(=O)C(=Cc2[nH]c(C)cc2C)c2ccccc12 |w:8.8| Show InChI InChI=1S/C17H16N2O3/c1-10-8-11(2)18-15(10)9-14-13-6-4-5-7-16(13)19(17(14)21)22-12(3)20/h4-9,18H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50171856

(3-[1-(3,5-Dimethyl-1H-pyrrol-2-yl)-meth-(Z)-yliden...)Show InChI InChI=1S/C15H14N2O2/c1-9-7-10(2)16-13(9)8-12-11-5-3-4-6-14(11)17(19)15(12)18/h3-8,16,19H,1-2H3/b12-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50171857

(3-[1-(4-Chloro-3,5-dimethyl-1H-pyrrol-2-yl)-meth-(...)Show InChI InChI=1S/C15H13ClN2O/c1-8-13(17-9(2)14(8)16)7-11-10-5-3-4-6-12(10)18-15(11)19/h3-7,17H,1-2H3,(H,18,19)/b11-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50171850
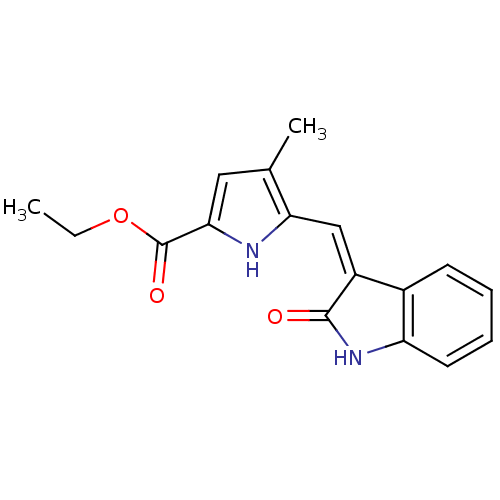
(4-Methyl-5-[2-oxo-1,2-dihydro-indol-(3Z)-ylideneme...)Show SMILES CCOC(=O)c1cc(C)c(\C=C2/C(=O)Nc3ccccc23)[nH]1 Show InChI InChI=1S/C17H16N2O3/c1-3-22-17(21)15-8-10(2)14(18-15)9-12-11-6-4-5-7-13(11)19-16(12)20/h4-9,18H,3H2,1-2H3,(H,19,20)/b12-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM253032

(US9487494, 53 (Compound 1056))Show SMILES C[C@@H](NC1CCCC(C1)c1ccc(cc1)C(O)=O)c1cccc2ccccc12 Show InChI InChI=1S/C25H27NO2/c1-17(23-11-5-7-19-6-2-3-10-24(19)23)26-22-9-4-8-21(16-22)18-12-14-20(15-13-18)25(27)28/h2-3,5-7,10-15,17,21-22,26H,4,8-9,16H2,1H3,(H,27,28)/t17-,21?,22?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM253030

(US9487494, 127 (Compound 1136))Show SMILES C[C@@H](N[C@H]1CCC[C@@H](C1)c1ccc(cc1)C(=O)NCCC(O)=O)c1cccc2ccccc12 Show InChI InChI=1S/C28H32N2O3/c1-19(25-11-5-7-21-6-2-3-10-26(21)25)30-24-9-4-8-23(18-24)20-12-14-22(15-13-20)28(33)29-17-16-27(31)32/h2-3,5-7,10-15,19,23-24,30H,4,8-9,16-18H2,1H3,(H,29,33)(H,31,32)/t19-,23+,24+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50171859

(4-Methyl-5-[2-oxo-1,2-dihydro-indol-(3Z)-ylideneme...)Show SMILES Cc1cc([nH]c1\C=C1/C(=O)Nc2ccccc12)C(=O)NCCO Show InChI InChI=1S/C17H17N3O3/c1-10-8-15(17(23)18-6-7-21)19-14(10)9-12-11-4-2-3-5-13(11)20-16(12)22/h2-5,8-9,19,21H,6-7H2,1H3,(H,18,23)(H,20,22)/b12-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50171854

(4-Methyl-5-[2-oxo-1,2-dihydro-indol-(3Z)-ylideneme...)Show InChI InChI=1S/C15H12N2O3/c1-8-6-13(15(19)20)16-12(8)7-10-9-4-2-3-5-11(9)17-14(10)18/h2-7,16H,1H3,(H,17,18)(H,19,20)/b10-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of KDR |
J Med Chem 48: 5412-4 (2005)
Article DOI: 10.1021/jm0504151
BindingDB Entry DOI: 10.7270/Q2319VD1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM253031

(US9487494, 106 (Compound 1115))Show SMILES C[C@@H](N[C@H]1CCC[C@@H](C1)c1ccc(cc1)C(=O)NCC(O)=O)c1cccc2ccccc12 Show InChI InChI=1S/C27H30N2O3/c1-18(24-11-5-7-20-6-2-3-10-25(20)24)29-23-9-4-8-22(16-23)19-12-14-21(15-13-19)27(32)28-17-26(30)31/h2-3,5-7,10-15,18,22-23,29H,4,8-9,16-17H2,1H3,(H,28,32)(H,30,31)/t18-,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM253028

(US9487494, 137 (Compound 1146))Show SMILES C[C@@H](N[C@H]1CCC[C@@H](C1)c1ccc(cc1)C(=O)N1CCC(CC1)C(O)=O)c1cccc2ccccc12 Show InChI InChI=1S/C31H36N2O3/c1-21(28-11-5-7-23-6-2-3-10-29(23)28)32-27-9-4-8-26(20-27)22-12-14-24(15-13-22)30(34)33-18-16-25(17-19-33)31(35)36/h2-3,5-7,10-15,21,25-27,32H,4,8-9,16-20H2,1H3,(H,35,36)/t21-,26+,27+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM253029
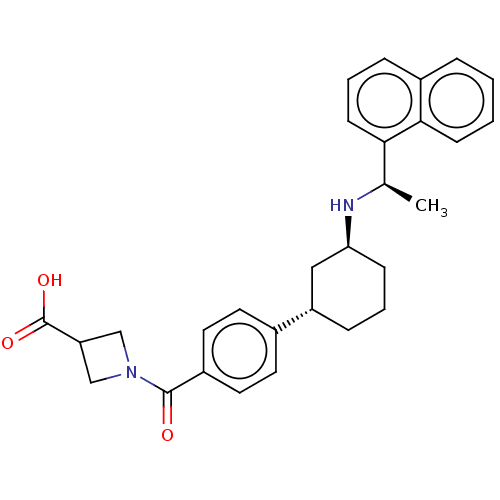
(US9487494, 133 (Compound 1142))Show SMILES C[C@@H](N[C@H]1CCC[C@@H](C1)c1ccc(cc1)C(=O)N1CC(C1)C(O)=O)c1cccc2ccccc12 Show InChI InChI=1S/C29H32N2O3/c1-19(26-11-5-7-21-6-2-3-10-27(21)26)30-25-9-4-8-23(16-25)20-12-14-22(15-13-20)28(32)31-17-24(18-31)29(33)34/h2-3,5-7,10-15,19,23-25,30H,4,8-9,16-18H2,1H3,(H,33,34)/t19-,23+,25+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM253025
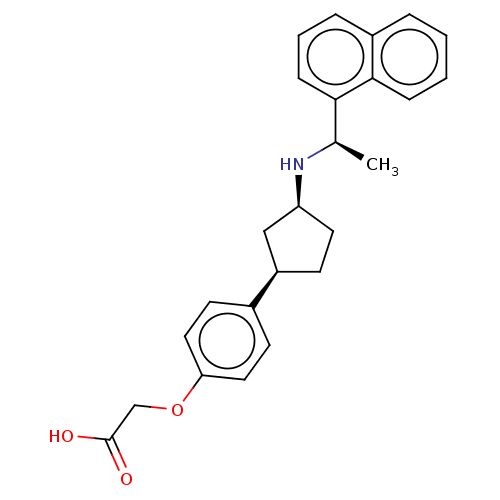
(US9487494, 171 (Compound 1186))Show SMILES C[C@@H](N[C@H]1CC[C@H](C1)c1ccc(OCC(O)=O)cc1)c1cccc2ccccc12 Show InChI InChI=1S/C25H27NO3/c1-17(23-8-4-6-19-5-2-3-7-24(19)23)26-21-12-9-20(15-21)18-10-13-22(14-11-18)29-16-25(27)28/h2-8,10-11,13-14,17,20-21,26H,9,12,15-16H2,1H3,(H,27,28)/t17-,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM253026
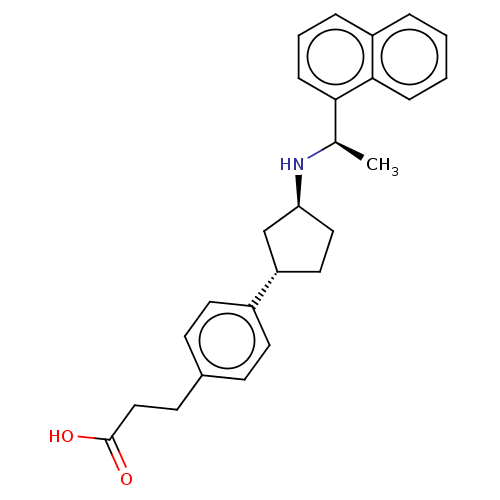
(US9487494, 175 (Compound 1190))Show SMILES C[C@@H](N[C@H]1CC[C@@H](C1)c1ccc(CCC(O)=O)cc1)c1cccc2ccccc12 Show InChI InChI=1S/C26H29NO2/c1-18(24-8-4-6-21-5-2-3-7-25(21)24)27-23-15-14-22(17-23)20-12-9-19(10-13-20)11-16-26(28)29/h2-10,12-13,18,22-23,27H,11,14-17H2,1H3,(H,28,29)/t18-,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM253027
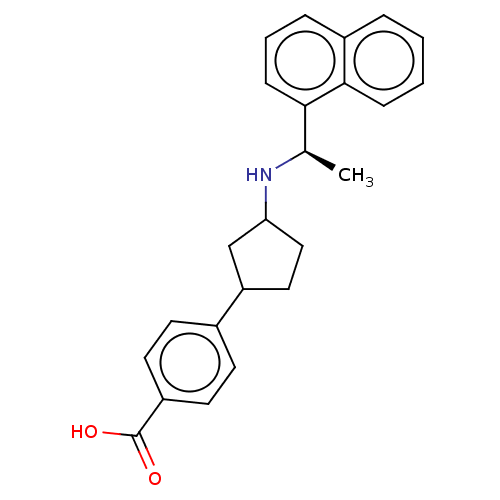
(US9487494, 299 (Compound 1338))Show SMILES C[C@@H](NC1CCC(C1)c1ccc(cc1)C(O)=O)c1cccc2ccccc12 Show InChI InChI=1S/C24H25NO2/c1-16(22-8-4-6-18-5-2-3-7-23(18)22)25-21-14-13-20(15-21)17-9-11-19(12-10-17)24(26)27/h2-12,16,20-21,25H,13-15H2,1H3,(H,26,27)/t16-,20?,21?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S
US Patent
| Assay Description
Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... |
US Patent US9487494 (2016)
BindingDB Entry DOI: 10.7270/Q21V5CX3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data