

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50274121 (CHEMBL4127092) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of wild-type HCV genotype 1b BK NS5B Cdelta55 RNA dependent RNA polymerase using RNA as substrate after 1 hr in presence of NTPs by [33P]-... | Bioorg Med Chem Lett 27: 5349-5352 (2017) Article DOI: 10.1016/j.bmcl.2017.06.064 BindingDB Entry DOI: 10.7270/Q2W95CSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50274122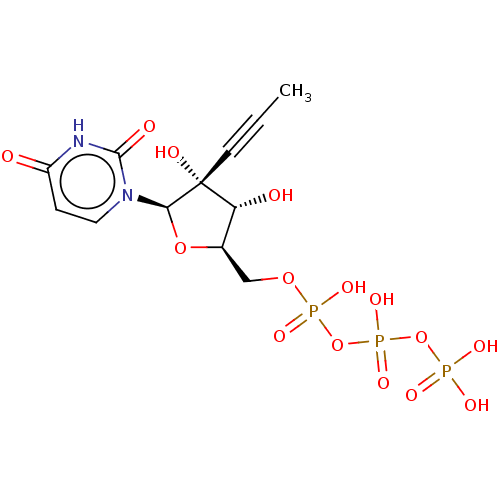 (CHEMBL4127819) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of wild-type HCV genotype 1b BK NS5B Cdelta55 RNA dependent RNA polymerase using RNA as substrate after 1 hr in presence of NTPs by [33P]-... | Bioorg Med Chem Lett 27: 5349-5352 (2017) Article DOI: 10.1016/j.bmcl.2017.06.064 BindingDB Entry DOI: 10.7270/Q2W95CSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224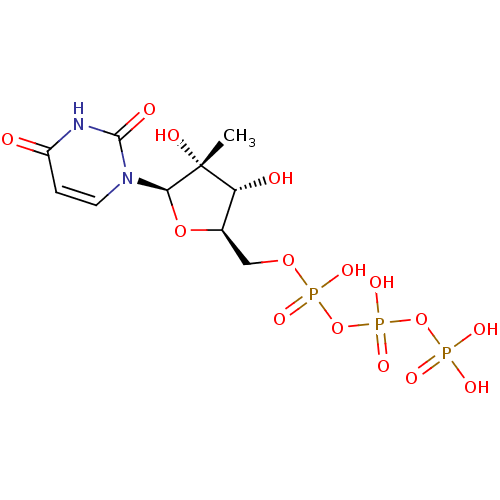 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of wild-type HCV genotype 1b BK NS5B Cdelta55 RNA dependent RNA polymerase using RNA as substrate after 1 hr in presence of NTPs by [33P]-... | Bioorg Med Chem Lett 27: 5349-5352 (2017) Article DOI: 10.1016/j.bmcl.2017.06.064 BindingDB Entry DOI: 10.7270/Q2W95CSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50333129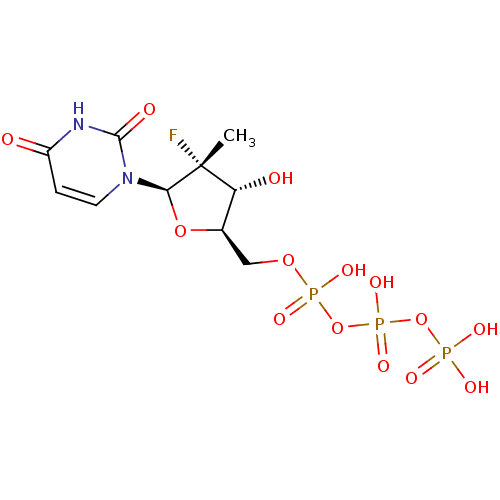 (((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of wild-type HCV genotype 1b BK NS5B Cdelta55 RNA dependent RNA polymerase using RNA as substrate after 1 hr in presence of NTPs by [33P]-... | Bioorg Med Chem Lett 27: 5349-5352 (2017) Article DOI: 10.1016/j.bmcl.2017.06.064 BindingDB Entry DOI: 10.7270/Q2W95CSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50274117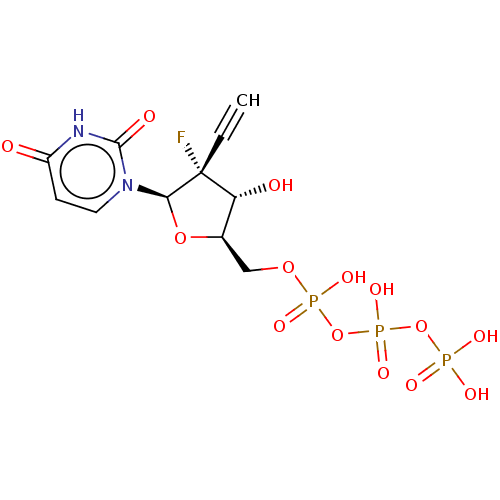 (CHEMBL4127921) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of wild-type HCV genotype 1b BK NS5B Cdelta55 RNA dependent RNA polymerase using RNA as substrate after 1 hr in presence of NTPs by [33P]-... | Bioorg Med Chem Lett 27: 5349-5352 (2017) Article DOI: 10.1016/j.bmcl.2017.06.064 BindingDB Entry DOI: 10.7270/Q2W95CSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50274123 (CHEMBL4129313) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of wild-type HCV genotype 1b BK NS5B Cdelta55 RNA dependent RNA polymerase using RNA as substrate after 1 hr in presence of NTPs by [33P]-... | Bioorg Med Chem Lett 27: 5349-5352 (2017) Article DOI: 10.1016/j.bmcl.2017.06.064 BindingDB Entry DOI: 10.7270/Q2W95CSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50444436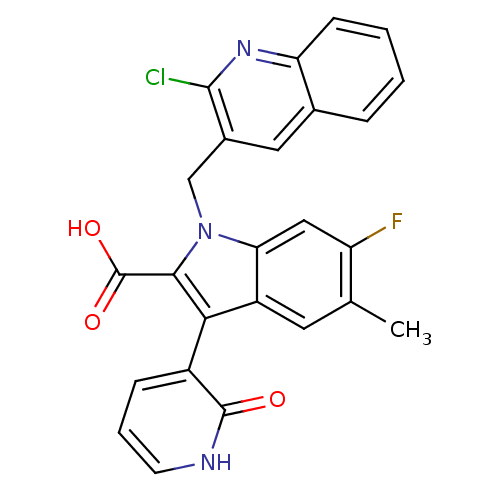 (CHEMBL3092124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2CJ in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50444436 (CHEMBL3092124) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP2D4 measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2CJ in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50444436 (CHEMBL3092124) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP2D4 measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50451636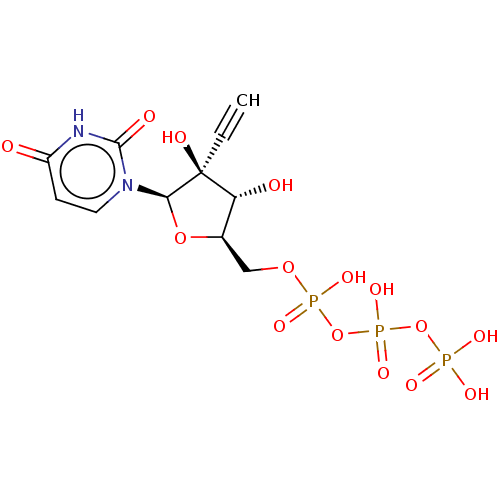 (CHEMBL4212840) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of wild-type HCV genotype 1b BK NS5B Cdelta55 RNA dependent RNA polymerase using RNA as substrate after 1 hr in presence of NTPs by [33P]-... | Bioorg Med Chem Lett 27: 5349-5352 (2017) Article DOI: 10.1016/j.bmcl.2017.06.064 BindingDB Entry DOI: 10.7270/Q2W95CSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||