 Found 112 hits with Last Name = 'fiebig' and Initial = 'hh'
Found 112 hits with Last Name = 'fiebig' and Initial = 'hh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551643
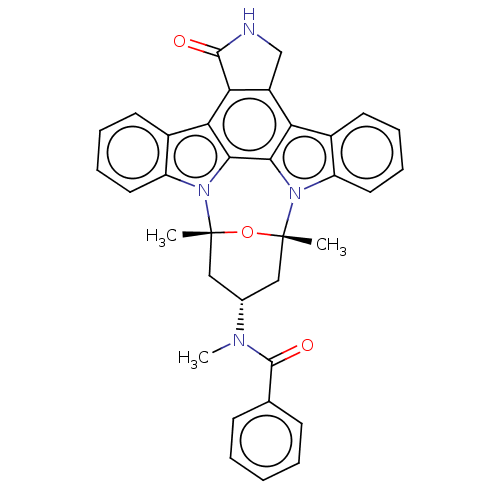
(CHEMBL4790597)Show SMILES CN([C@H]1C[C@]2(C)O[C@](C)(C1)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13)C(=O)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551643

(CHEMBL4790597)Show SMILES CN([C@H]1C[C@]2(C)O[C@](C)(C1)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13)C(=O)c1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551644
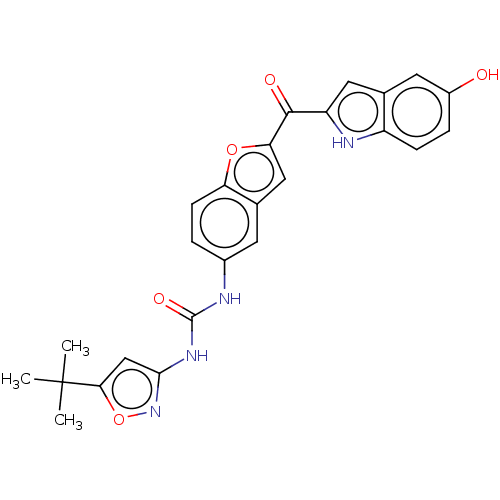
(CHEMBL4745937)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc3oc(cc3c2)C(=O)c2cc3cc(O)ccc3[nH]2)no1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551649
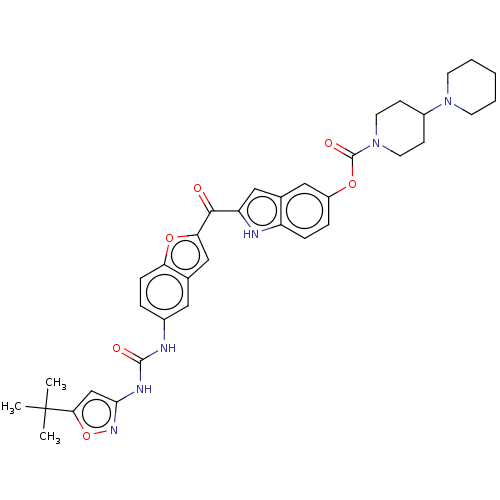
(CHEMBL4765060)Show SMILES Cl.CC(C)(C)c1cc(NC(=O)Nc2ccc3oc(cc3c2)C(=O)c2cc3cc(OC(=O)N4CCC(CC4)N4CCCCC4)ccc3[nH]2)no1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551649

(CHEMBL4765060)Show SMILES Cl.CC(C)(C)c1cc(NC(=O)Nc2ccc3oc(cc3c2)C(=O)c2cc3cc(OC(=O)N4CCC(CC4)N4CCCCC4)ccc3[nH]2)no1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551644

(CHEMBL4745937)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc3oc(cc3c2)C(=O)c2cc3cc(O)ccc3[nH]2)no1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359993
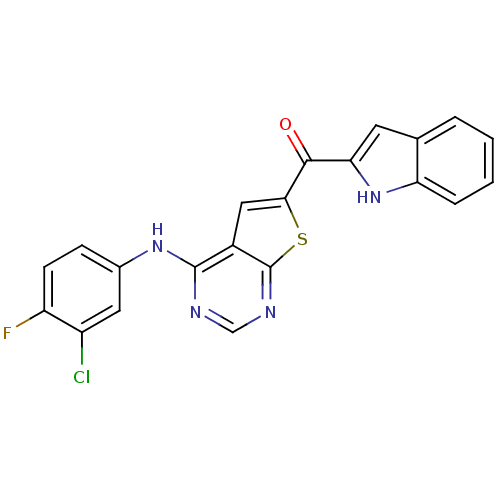
(CHEMBL1928291)Show SMILES Fc1ccc(Nc2ncnc3sc(cc23)C(=O)c2cc3ccccc3[nH]2)cc1Cl Show InChI InChI=1S/C21H12ClFN4OS/c22-14-8-12(5-6-15(14)23)26-20-13-9-18(29-21(13)25-10-24-20)19(28)17-7-11-3-1-2-4-16(11)27-17/h1-10,27H,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359997
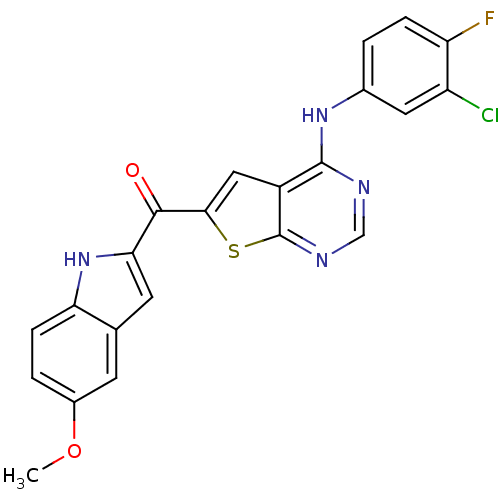
(CHEMBL1928312)Show SMILES COc1ccc2[nH]c(cc2c1)C(=O)c1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2s1 Show InChI InChI=1S/C22H14ClFN4O2S/c1-30-13-3-5-17-11(6-13)7-18(28-17)20(29)19-9-14-21(25-10-26-22(14)31-19)27-12-2-4-16(24)15(23)8-12/h2-10,28H,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359998
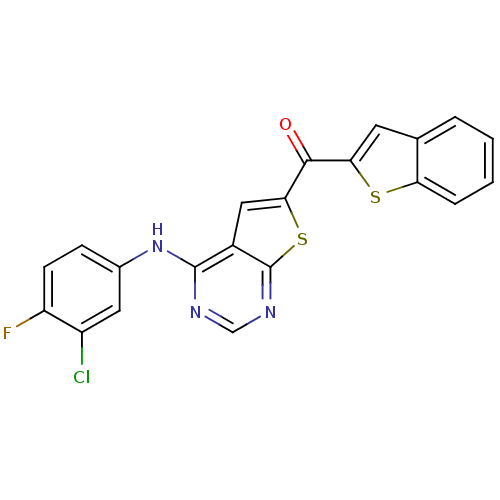
(CHEMBL1928315)Show SMILES Fc1ccc(Nc2ncnc3sc(cc23)C(=O)c2cc3ccccc3s2)cc1Cl Show InChI InChI=1S/C21H11ClFN3OS2/c22-14-8-12(5-6-15(14)23)26-20-13-9-18(29-21(13)25-10-24-20)19(27)17-7-11-3-1-2-4-16(11)28-17/h1-10H,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551647

(CHEMBL4755980)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc3[nH]c(cc3c2)C(=O)c2cc3cc(O)ccc3o2)no1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551650
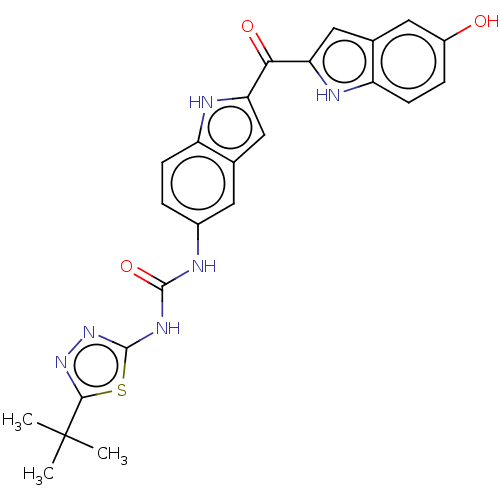
(CHEMBL4754982)Show SMILES CC(C)(C)c1nnc(NC(=O)Nc2ccc3[nH]c(cc3c2)C(=O)c2cc3cc(O)ccc3[nH]2)s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359999
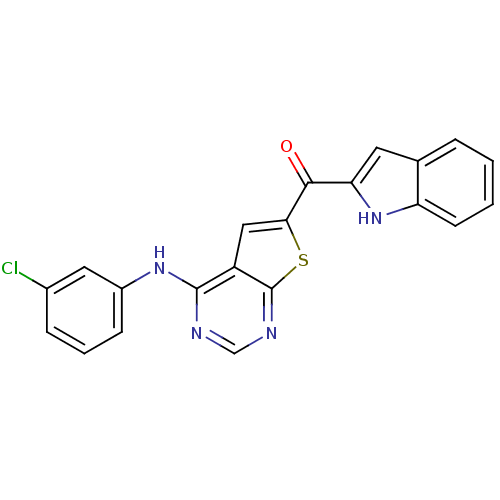
(CHEMBL1928309)Show SMILES Clc1cccc(Nc2ncnc3sc(cc23)C(=O)c2cc3ccccc3[nH]2)c1 Show InChI InChI=1S/C21H13ClN4OS/c22-13-5-3-6-14(9-13)25-20-15-10-18(28-21(15)24-11-23-20)19(27)17-8-12-4-1-2-7-16(12)26-17/h1-11,26H,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359995
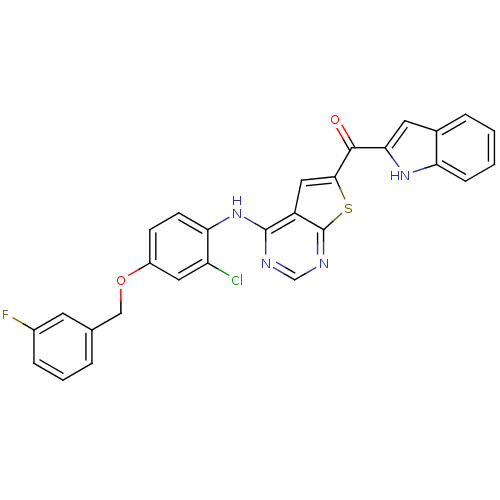
(CHEMBL1928301)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4sc(cc34)C(=O)c3cc4ccccc4[nH]3)c(Cl)c2)c1 Show InChI InChI=1S/C28H18ClFN4O2S/c29-21-12-19(36-14-16-4-3-6-18(30)10-16)8-9-23(21)34-27-20-13-25(37-28(20)32-15-31-27)26(35)24-11-17-5-1-2-7-22(17)33-24/h1-13,15,33H,14H2,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359994
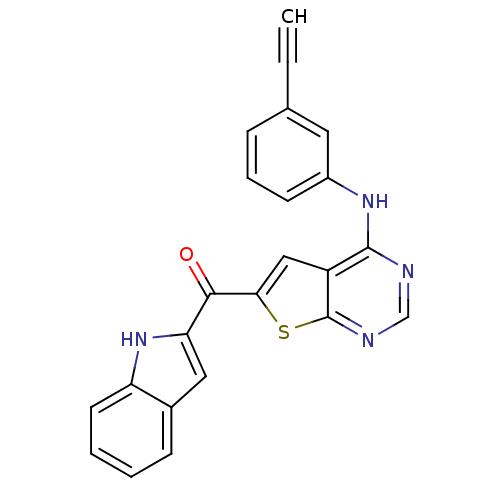
(CHEMBL1928293)Show SMILES O=C(c1cc2ccccc2[nH]1)c1cc2c(Nc3cccc(c3)C#C)ncnc2s1 Show InChI InChI=1S/C23H14N4OS/c1-2-14-6-5-8-16(10-14)26-22-17-12-20(29-23(17)25-13-24-22)21(28)19-11-15-7-3-4-9-18(15)27-19/h1,3-13,27H,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359992
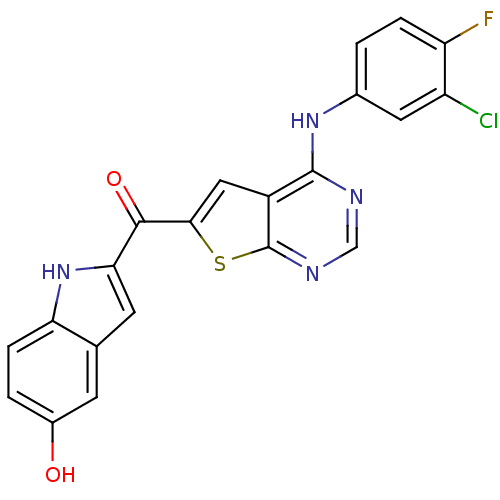
(CHEMBL1928311)Show SMILES Oc1ccc2[nH]c(cc2c1)C(=O)c1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2s1 Show InChI InChI=1S/C21H12ClFN4O2S/c22-14-7-11(1-3-15(14)23)26-20-13-8-18(30-21(13)25-9-24-20)19(29)17-6-10-5-12(28)2-4-16(10)27-17/h1-9,27-28H,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551650

(CHEMBL4754982)Show SMILES CC(C)(C)c1nnc(NC(=O)Nc2ccc3[nH]c(cc3c2)C(=O)c2cc3cc(O)ccc3[nH]2)s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551648
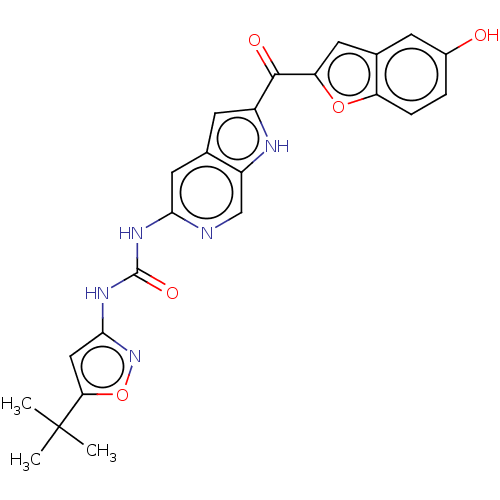
(CHEMBL4740264)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2cc3cc([nH]c3cn2)C(=O)c2cc3cc(O)ccc3o2)no1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359996
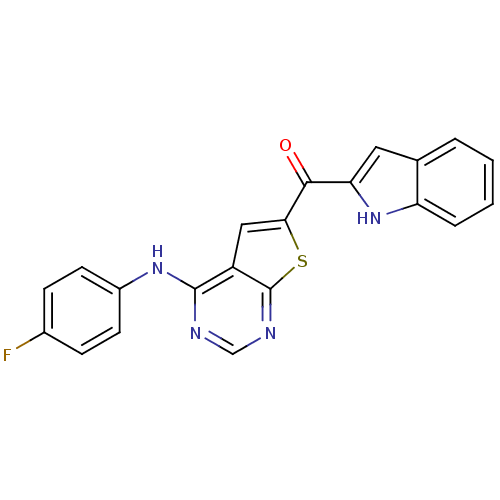
(CHEMBL1928310)Show SMILES Fc1ccc(Nc2ncnc3sc(cc23)C(=O)c2cc3ccccc3[nH]2)cc1 Show InChI InChI=1S/C21H13FN4OS/c22-13-5-7-14(8-6-13)25-20-15-10-18(28-21(15)24-11-23-20)19(27)17-9-12-3-1-2-4-16(12)26-17/h1-11,26H,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of Erbb2 using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551646
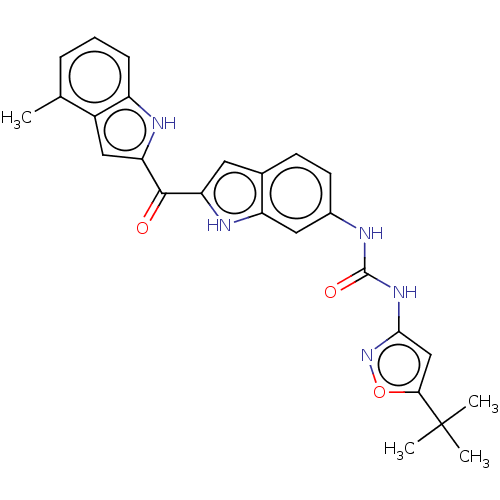
(CHEMBL4751666)Show SMILES Cc1cccc2[nH]c(cc12)C(=O)c1cc2ccc(NC(=O)Nc3cc(on3)C(C)(C)C)cc2[nH]1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359992

(CHEMBL1928311)Show SMILES Oc1ccc2[nH]c(cc2c1)C(=O)c1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2s1 Show InChI InChI=1S/C21H12ClFN4O2S/c22-14-7-11(1-3-15(14)23)26-20-13-8-18(30-21(13)25-9-24-20)19(29)17-6-10-5-12(28)2-4-16(10)27-17/h1-9,27-28H,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR autophosphorylation in EGF-stimulated human A431 cells after 1 hr by immunoblotting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551645
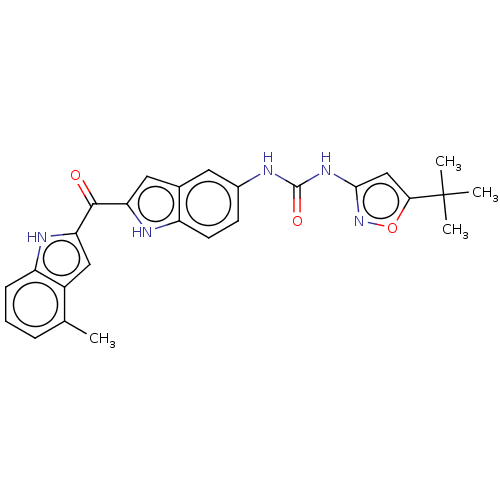
(CHEMBL4764595)Show SMILES Cc1cccc2[nH]c(cc12)C(=O)c1cc2cc(NC(=O)Nc3cc(on3)C(C)(C)C)ccc2[nH]1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551647

(CHEMBL4755980)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc3[nH]c(cc3c2)C(=O)c2cc3cc(O)ccc3o2)no1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50359999

(CHEMBL1928309)Show SMILES Clc1cccc(Nc2ncnc3sc(cc23)C(=O)c2cc3ccccc3[nH]2)c1 Show InChI InChI=1S/C21H13ClN4OS/c22-13-5-3-6-14(9-13)25-20-15-10-18(28-21(15)24-11-23-20)19(27)17-8-12-4-1-2-7-16(12)26-17/h1-11,26H,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of EGFR autophosphorylation in EGF-stimulated human A431 cells after 1 hr by immunoblotting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of Erbb2 using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107672
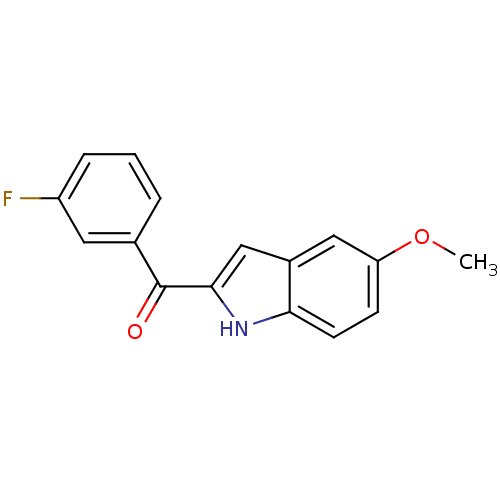
((3-Fluoro-phenyl)-(5-methoxy-1H-indol-2-yl)-methan...)Show InChI InChI=1S/C16H12FNO2/c1-20-13-5-6-14-11(8-13)9-15(18-14)16(19)10-3-2-4-12(17)7-10/h2-9,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107658
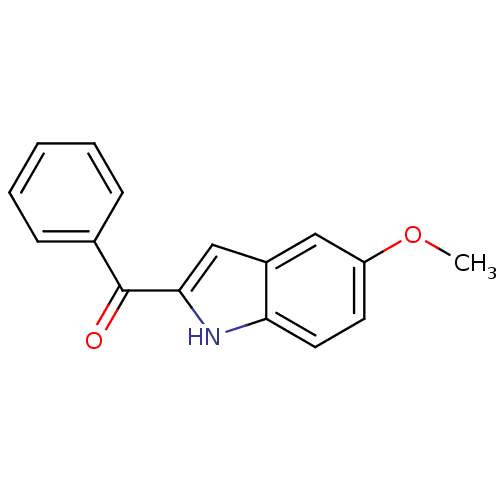
((5-Methoxy-1H-indol-2-yl)-phenyl-methanone | (5-me...)Show InChI InChI=1S/C16H13NO2/c1-19-13-7-8-14-12(9-13)10-15(17-14)16(18)11-5-3-2-4-6-11/h2-10,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107678
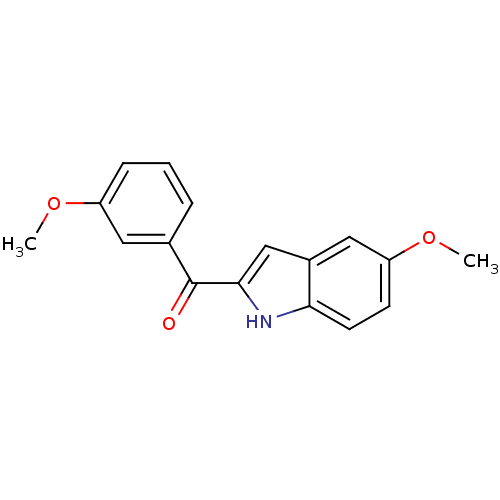
((5-Methoxy-1H-indol-2-yl)-(3-methoxy-phenyl)-metha...)Show InChI InChI=1S/C17H15NO3/c1-20-13-5-3-4-11(8-13)17(19)16-10-12-9-14(21-2)6-7-15(12)18-16/h3-10,18H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107677
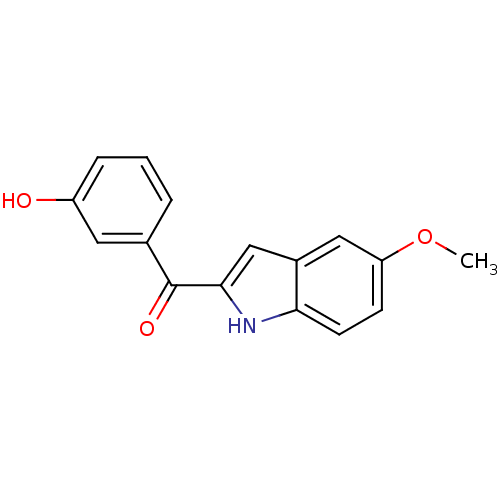
((3-Hydroxy-phenyl)-(5-methoxy-1H-indol-2-yl)-metha...)Show InChI InChI=1S/C16H13NO3/c1-20-13-5-6-14-11(8-13)9-15(17-14)16(19)10-3-2-4-12(18)7-10/h2-9,17-18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107656

((3,5-Dimethoxy-phenyl)-(5-methoxy-1H-indol-2-yl)-m...)Show InChI InChI=1S/C18H17NO4/c1-21-13-4-5-16-11(6-13)9-17(19-16)18(20)12-7-14(22-2)10-15(8-12)23-3/h4-10,19H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107676

((3,4-Dimethoxy-phenyl)-(5-methoxy-1H-indol-2-yl)-m...)Show InChI InChI=1S/C18H17NO4/c1-21-13-5-6-14-12(8-13)9-15(19-14)18(20)11-4-7-16(22-2)17(10-11)23-3/h4-10,19H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 using poly(Glu,Tyr)4:1 as substrate and [gamma33P]ATP after 60 mins by scintillation counting |
Bioorg Med Chem 20: 125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.11.023
BindingDB Entry DOI: 10.7270/Q2J966SR |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107674

(Butyric acid 3-(5-methoxy-1H-indole-2-carbonyl)-ph...)Show SMILES CCCC(=O)Oc1cccc(c1)C(=O)c1cc2cc(OC)ccc2[nH]1 Show InChI InChI=1S/C20H19NO4/c1-3-5-19(22)25-16-7-4-6-13(10-16)20(23)18-12-14-11-15(24-2)8-9-17(14)21-18/h4,6-12,21H,3,5H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107664
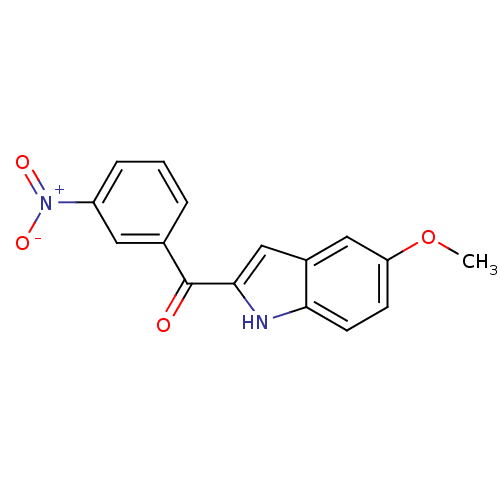
((5-Methoxy-1H-indol-2-yl)-(3-nitro-phenyl)-methano...)Show SMILES COc1ccc2[nH]c(cc2c1)C(=O)c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C16H12N2O4/c1-22-13-5-6-14-11(8-13)9-15(17-14)16(19)10-3-2-4-12(7-10)18(20)21/h2-9,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107693

((5-Methyl-1H-indol-2-yl)-(3,4,5-trimethoxy-phenyl)...)Show InChI InChI=1S/C19H19NO4/c1-11-5-6-14-12(7-11)8-15(20-14)18(21)13-9-16(22-2)19(24-4)17(10-13)23-3/h5-10,20H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aromatase |
J Nat Prod 70: 353-60 (2007)
Article DOI: 10.1021/np060505o
BindingDB Entry DOI: 10.7270/Q2930STQ |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107662

((3-Amino-phenyl)-(5-methoxy-1H-indol-2-yl)-methano...)Show InChI InChI=1S/C16H14N2O2/c1-20-13-5-6-14-11(8-13)9-15(18-14)16(19)10-3-2-4-12(17)7-10/h2-9,18H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107651

((5-Methoxy-1H-indol-2-yl)-(3,4,5-trimethoxy-phenyl...)Show SMILES COc1ccc2[nH]c(cc2c1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H19NO5/c1-22-13-5-6-14-11(7-13)8-15(20-14)18(21)12-9-16(23-2)19(25-4)17(10-12)24-3/h5-10,20H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107663
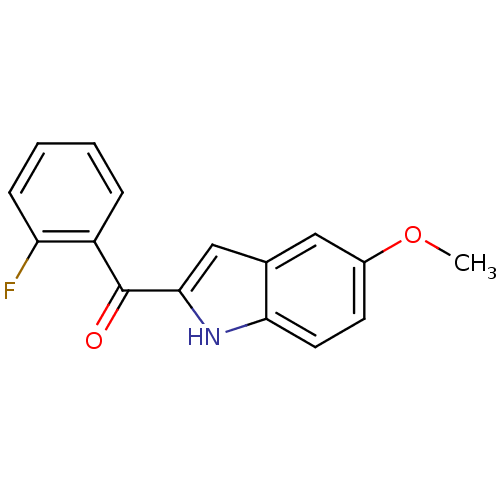
((2-Fluoro-phenyl)-(5-methoxy-1H-indol-2-yl)-methan...)Show InChI InChI=1S/C16H12FNO2/c1-20-11-6-7-14-10(8-11)9-15(18-14)16(19)12-4-2-3-5-13(12)17/h2-9,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107670

((5-Methoxy-1H-indol-2-yl)-(2-methoxy-phenyl)-metha...)Show InChI InChI=1S/C17H15NO3/c1-20-12-7-8-14-11(9-12)10-15(18-14)17(19)13-5-3-4-6-16(13)21-2/h3-10,18H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107680

((5-Methoxy-1H-indol-2-yl)-p-tolyl-methanone | CHEM...)Show InChI InChI=1S/C17H15NO2/c1-11-3-5-12(6-4-11)17(19)16-10-13-9-14(20-2)7-8-15(13)18-16/h3-10,18H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107671

((5-Methoxy-1H-indol-2-yl)-(3-trifluoromethyl-pheny...)Show SMILES COc1ccc2[nH]c(cc2c1)C(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C17H12F3NO2/c1-23-13-5-6-14-11(8-13)9-15(21-14)16(22)10-3-2-4-12(7-10)17(18,19)20/h2-9,21H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107682
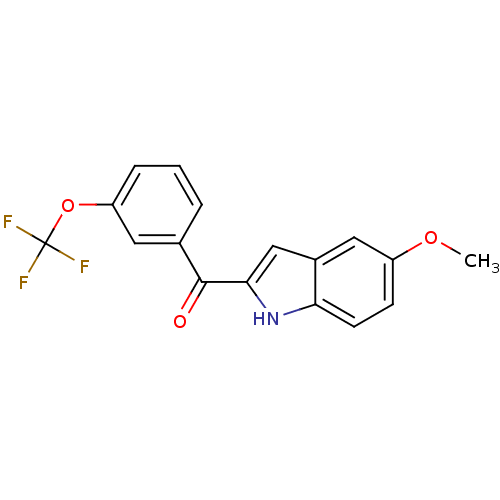
((5-Methoxy-1H-indol-2-yl)-(3-trifluoromethoxy-phen...)Show SMILES COc1ccc2[nH]c(cc2c1)C(=O)c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C17H12F3NO3/c1-23-12-5-6-14-11(8-12)9-15(21-14)16(22)10-3-2-4-13(7-10)24-17(18,19)20/h2-9,21H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization. |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551646

(CHEMBL4751666)Show SMILES Cc1cccc2[nH]c(cc12)C(=O)c1cc2ccc(NC(=O)Nc3cc(on3)C(C)(C)C)cc2[nH]1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50551648

(CHEMBL4740264)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2cc3cc([nH]c3cn2)C(=O)c2cc3cc(O)ccc3o2)no1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112232
BindingDB Entry DOI: 10.7270/Q25H7KWP |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50107673

((3-Difluoromethylsulfanyl-phenyl)-(5-methoxy-1H-in...)Show InChI InChI=1S/C17H13F2NO2S/c1-22-12-5-6-14-11(7-12)9-15(20-14)16(21)10-3-2-4-13(8-10)23-17(18)19/h2-9,17,20H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of tubulin polymerization |
J Med Chem 44: 4535-53 (2001)
BindingDB Entry DOI: 10.7270/Q2BZ65CN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data