 Found 65 hits with Last Name = 'fielden' and Initial = 'm'
Found 65 hits with Last Name = 'fielden' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446517

(CHEMBL3110092)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)[C@H](C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C20H29N5O3/c1-6-27-17-7-8-18(13(2)11-17)24-9-10-25(14(3)12-24)20-22-19(28-23-20)15(4)21-16(5)26/h7-8,11,14-15H,6,9-10,12H2,1-5H3,(H,21,26)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446514

(CHEMBL3110076)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C2(CCC2)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C22H31N5O3/c1-5-29-18-7-8-19(15(2)13-18)26-11-12-27(16(3)14-26)21-23-20(30-25-21)22(9-6-10-22)24-17(4)28/h7-8,13,16H,5-6,9-12,14H2,1-4H3,(H,24,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446515
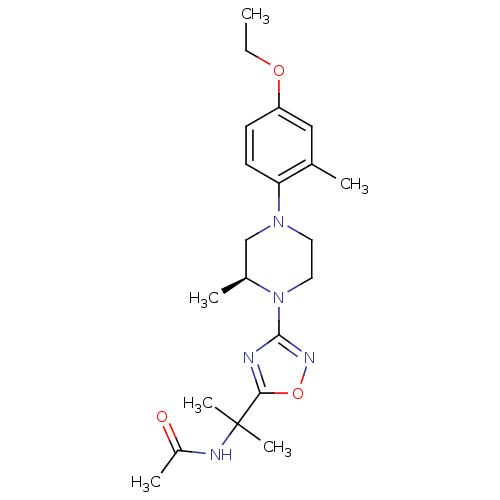
(CHEMBL3110075)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C(C)(C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C21H31N5O3/c1-7-28-17-8-9-18(14(2)12-17)25-10-11-26(15(3)13-25)20-22-19(29-24-20)21(5,6)23-16(4)27/h8-9,12,15H,7,10-11,13H2,1-6H3,(H,23,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446516
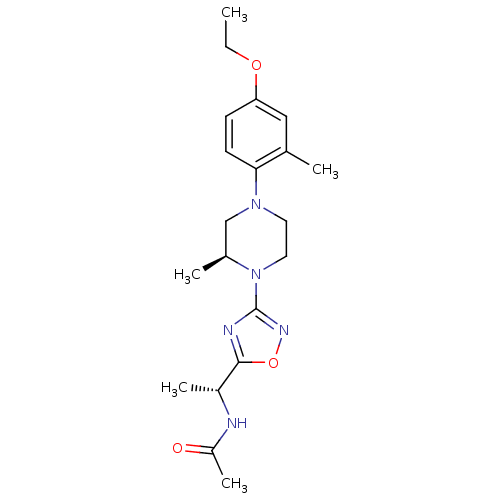
(CHEMBL3110093)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)[C@@H](C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C20H29N5O3/c1-6-27-17-7-8-18(13(2)11-17)24-9-10-25(14(3)12-24)20-22-19(28-23-20)15(4)21-16(5)26/h7-8,11,14-15H,6,9-10,12H2,1-5H3,(H,21,26)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446517

(CHEMBL3110092)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)[C@H](C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C20H29N5O3/c1-6-27-17-7-8-18(13(2)11-17)24-9-10-25(14(3)12-24)20-22-19(28-23-20)15(4)21-16(5)26/h7-8,11,14-15H,6,9-10,12H2,1-5H3,(H,21,26)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446528
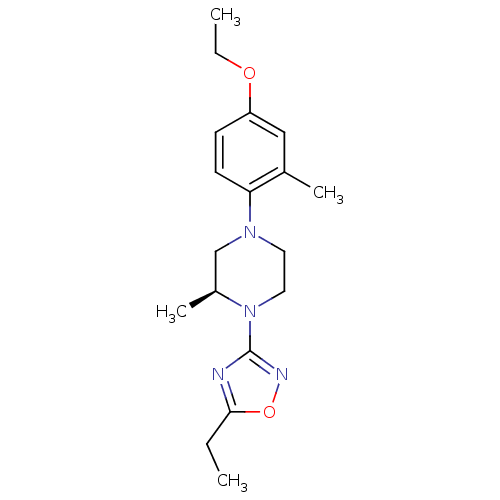
(CHEMBL3110081)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C18H26N4O2/c1-5-17-19-18(20-24-17)22-10-9-21(12-14(22)4)16-8-7-15(23-6-2)11-13(16)3/h7-8,11,14H,5-6,9-10,12H2,1-4H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446528

(CHEMBL3110081)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C18H26N4O2/c1-5-17-19-18(20-24-17)22-10-9-21(12-14(22)4)16-8-7-15(23-6-2)11-13(16)3/h7-8,11,14H,5-6,9-10,12H2,1-4H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446516

(CHEMBL3110093)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)[C@@H](C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C20H29N5O3/c1-6-27-17-7-8-18(13(2)11-17)24-9-10-25(14(3)12-24)20-22-19(28-23-20)15(4)21-16(5)26/h7-8,11,14-15H,6,9-10,12H2,1-5H3,(H,21,26)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446514

(CHEMBL3110076)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C2(CCC2)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C22H31N5O3/c1-5-29-18-7-8-19(15(2)13-18)26-11-12-27(16(3)14-26)21-23-20(30-25-21)22(9-6-10-22)24-17(4)28/h7-8,13,16H,5-6,9-12,14H2,1-4H3,(H,24,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446518
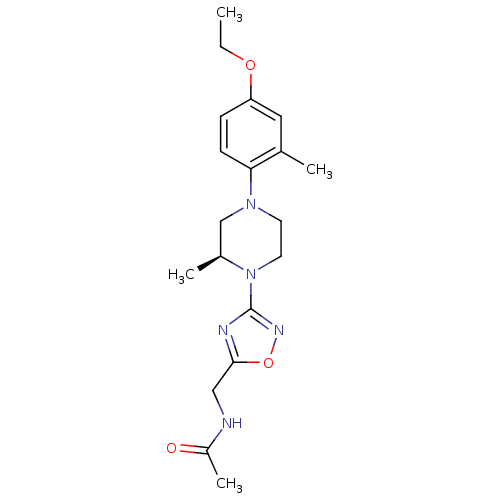
(CHEMBL3110091)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CNC(C)=O)n2)c(C)c1 |r| Show InChI InChI=1S/C19H27N5O3/c1-5-26-16-6-7-17(13(2)10-16)23-8-9-24(14(3)12-23)19-21-18(27-22-19)11-20-15(4)25/h6-7,10,14H,5,8-9,11-12H2,1-4H3,(H,20,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446535
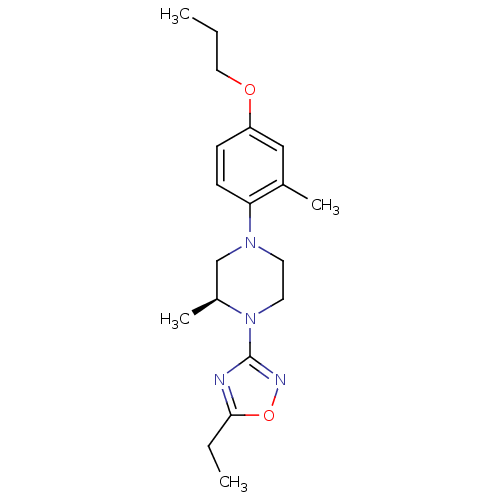
(CHEMBL3110098)Show SMILES CCCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-11-24-16-7-8-17(14(3)12-16)22-9-10-23(15(4)13-22)19-20-18(6-2)25-21-19/h7-8,12,15H,5-6,9-11,13H2,1-4H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Mus musculus) | BDBM50446514

(CHEMBL3110076)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C2(CCC2)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C22H31N5O3/c1-5-29-18-7-8-19(15(2)13-18)26-11-12-27(16(3)14-26)21-23-20(30-25-21)22(9-6-10-22)24-17(4)28/h7-8,13,16H,5-6,9-12,14H2,1-4H3,(H,24,28)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ACC2 |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446512
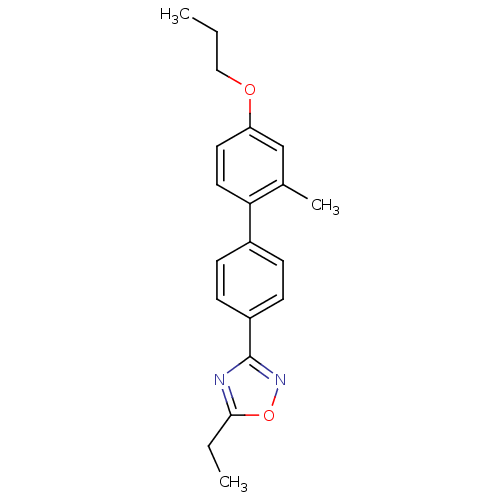
(CHEMBL3110095)Show InChI InChI=1S/C20H22N2O2/c1-4-12-23-17-10-11-18(14(3)13-17)15-6-8-16(9-7-15)20-21-19(5-2)24-22-20/h6-11,13H,4-5,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446512

(CHEMBL3110095)Show InChI InChI=1S/C20H22N2O2/c1-4-12-23-17-10-11-18(14(3)13-17)15-6-8-16(9-7-15)20-21-19(5-2)24-22-20/h6-11,13H,4-5,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446511
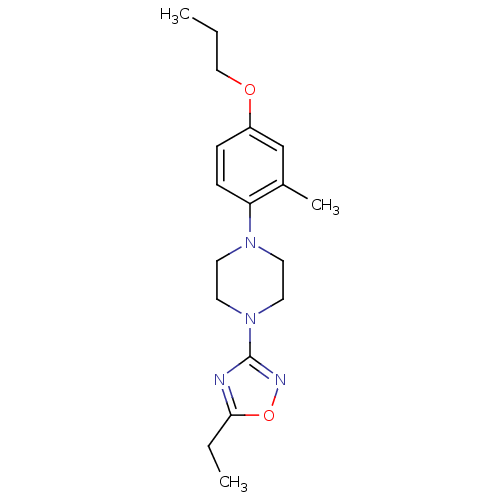
(CHEMBL3110096)Show InChI InChI=1S/C18H26N4O2/c1-4-12-23-15-6-7-16(14(3)13-15)21-8-10-22(11-9-21)18-19-17(5-2)24-20-18/h6-7,13H,4-5,8-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446520
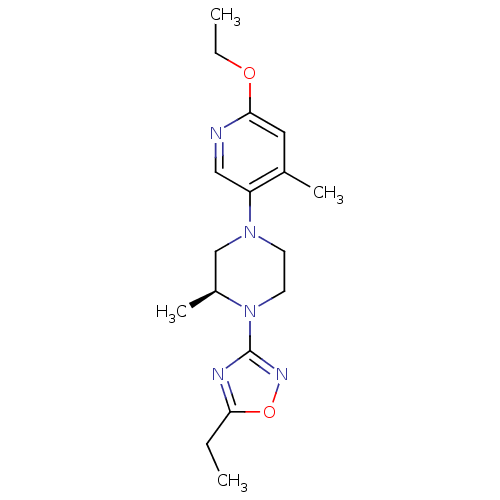
(CHEMBL3110089)Show SMILES CCOc1cc(C)c(cn1)N1CCN([C@@H](C)C1)c1noc(CC)n1 |r| Show InChI InChI=1S/C17H25N5O2/c1-5-15-19-17(20-24-15)22-8-7-21(11-13(22)4)14-10-18-16(23-6-2)9-12(14)3/h9-10,13H,5-8,11H2,1-4H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446530
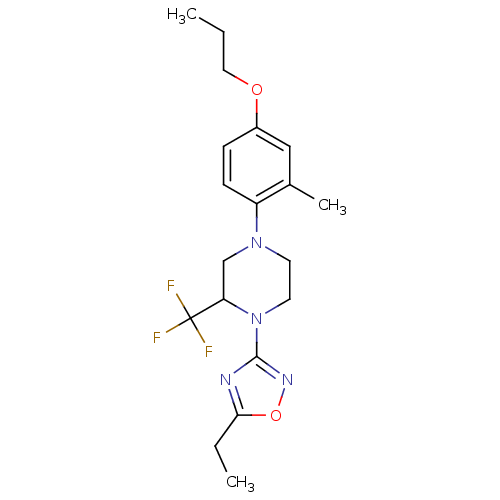
(CHEMBL3110079)Show SMILES CCCOc1ccc(N2CCN(C(C2)C(F)(F)F)c2noc(CC)n2)c(C)c1 Show InChI InChI=1S/C19H25F3N4O2/c1-4-10-27-14-6-7-15(13(3)11-14)25-8-9-26(16(12-25)19(20,21)22)18-23-17(5-2)28-24-18/h6-7,11,16H,4-5,8-10,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446536
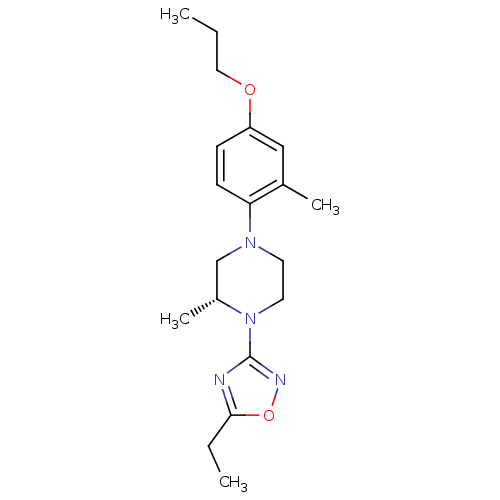
(CHEMBL3110097)Show SMILES CCCOc1ccc(N2CCN([C@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-11-24-16-7-8-17(14(3)12-16)22-9-10-23(15(4)13-22)19-20-18(6-2)25-21-19/h7-8,12,15H,5-6,9-11,13H2,1-4H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446514

(CHEMBL3110076)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C2(CCC2)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C22H31N5O3/c1-5-29-18-7-8-19(15(2)13-18)26-11-12-27(16(3)14-26)21-23-20(30-25-21)22(9-6-10-22)24-17(4)28/h7-8,13,16H,5-6,9-12,14H2,1-4H3,(H,24,28)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446522
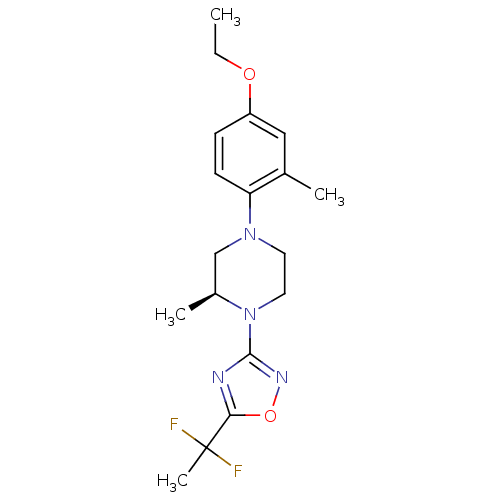
(CHEMBL3110087)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C(C)(F)F)c(C)c1 |r| Show InChI InChI=1S/C18H24F2N4O2/c1-5-25-14-6-7-15(12(2)10-14)23-8-9-24(13(3)11-23)17-21-16(26-22-17)18(4,19)20/h6-7,10,13H,5,8-9,11H2,1-4H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446532
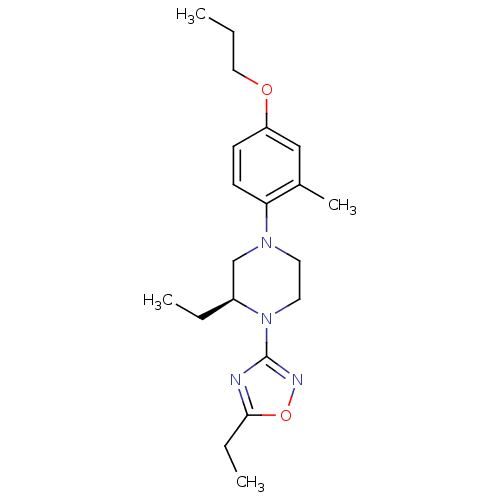
(CHEMBL3110077)Show SMILES CCCOc1ccc(N2CCN([C@@H](CC)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C20H30N4O2/c1-5-12-25-17-8-9-18(15(4)13-17)23-10-11-24(16(6-2)14-23)20-21-19(7-3)26-22-20/h8-9,13,16H,5-7,10-12,14H2,1-4H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446524

(CHEMBL3110085)Show SMILES CCCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(Cl)c1 |r| Show InChI InChI=1S/C18H25ClN4O2/c1-4-10-24-14-6-7-16(15(19)11-14)22-8-9-23(13(3)12-22)18-20-17(5-2)25-21-18/h6-7,11,13H,4-5,8-10,12H2,1-3H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446523
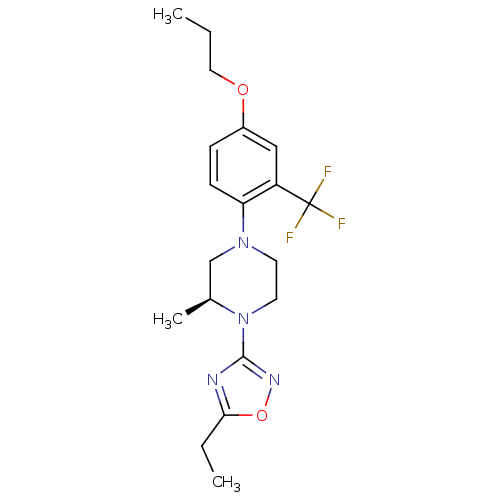
(CHEMBL3110086)Show SMILES CCCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H25F3N4O2/c1-4-10-27-14-6-7-16(15(11-14)19(20,21)22)25-8-9-26(13(3)12-25)18-23-17(5-2)28-24-18/h6-7,11,13H,4-5,8-10,12H2,1-3H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446521
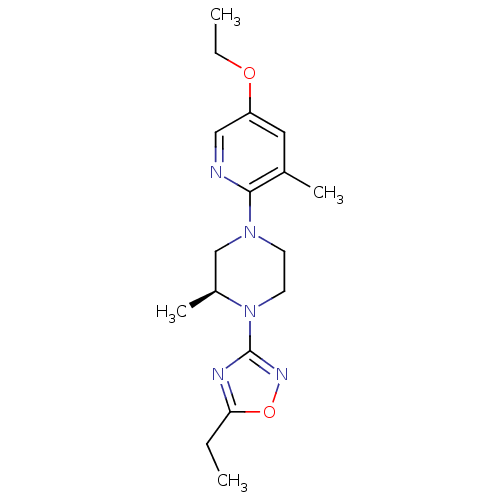
(CHEMBL3110088)Show SMILES CCOc1cnc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C17H25N5O2/c1-5-15-19-17(20-24-15)22-8-7-21(11-13(22)4)16-12(3)9-14(10-18-16)23-6-2/h9-10,13H,5-8,11H2,1-4H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446518

(CHEMBL3110091)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CNC(C)=O)n2)c(C)c1 |r| Show InChI InChI=1S/C19H27N5O3/c1-5-26-16-6-7-17(13(2)10-16)23-8-9-24(14(3)12-23)19-21-18(27-22-19)11-20-15(4)25/h6-7,10,14H,5,8-9,11-12H2,1-4H3,(H,20,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446527
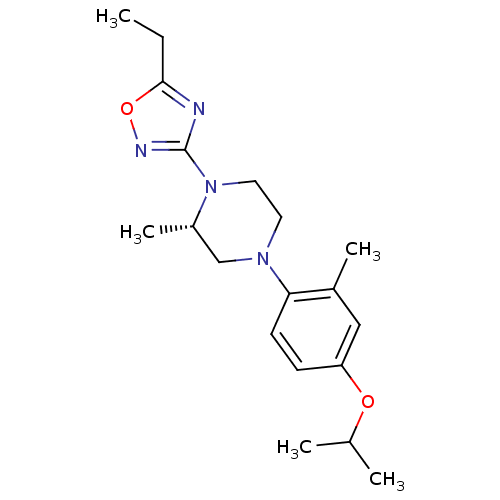
(CHEMBL3110082)Show SMILES CCc1nc(no1)N1CCN(C[C@@H]1C)c1ccc(OC(C)C)cc1C |r| Show InChI InChI=1S/C19H28N4O2/c1-6-18-20-19(21-25-18)23-10-9-22(12-15(23)5)17-8-7-16(11-14(17)4)24-13(2)3/h7-8,11,13,15H,6,9-10,12H2,1-5H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446531
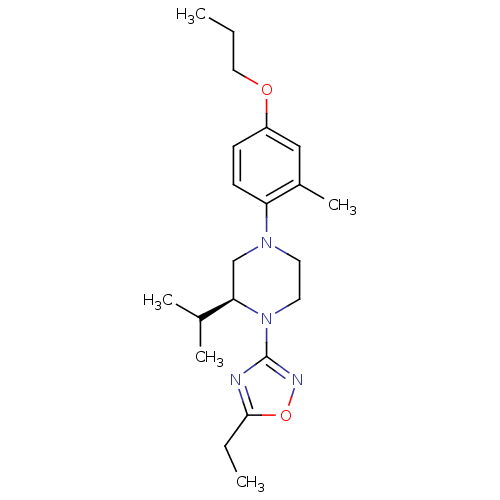
(CHEMBL3110078)Show SMILES CCCOc1ccc(N2CCN([C@H](C2)C(C)C)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C21H32N4O2/c1-6-12-26-17-8-9-18(16(5)13-17)24-10-11-25(19(14-24)15(3)4)21-22-20(7-2)27-23-21/h8-9,13,15,19H,6-7,10-12,14H2,1-5H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446515

(CHEMBL3110075)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C(C)(C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C21H31N5O3/c1-7-28-17-8-9-18(14(2)12-17)25-10-11-26(15(3)13-25)20-22-19(29-24-20)21(5,6)23-16(4)27/h8-9,12,15H,7,10-11,13H2,1-6H3,(H,23,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446519
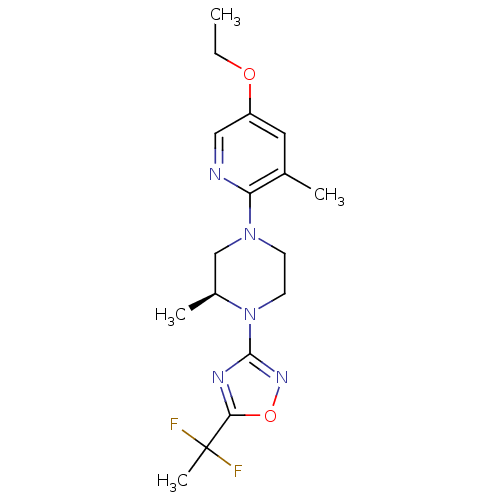
(CHEMBL3110090)Show SMILES CCOc1cnc(N2CCN([C@@H](C)C2)c2noc(n2)C(C)(F)F)c(C)c1 |r| Show InChI InChI=1S/C17H23F2N5O2/c1-5-25-13-8-11(2)14(20-9-13)23-6-7-24(12(3)10-23)16-21-15(26-22-16)17(4,18)19/h8-9,12H,5-7,10H2,1-4H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446513
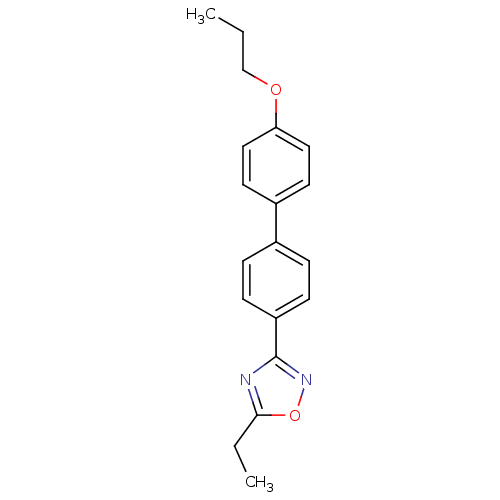
(CHEMBL3110094)Show InChI InChI=1S/C19H20N2O2/c1-3-13-22-17-11-9-15(10-12-17)14-5-7-16(8-6-14)19-20-18(4-2)23-21-19/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 359 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446517

(CHEMBL3110092)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)[C@H](C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C20H29N5O3/c1-6-27-17-7-8-18(13(2)11-17)24-9-10-25(14(3)12-24)20-22-19(28-23-20)15(4)21-16(5)26/h7-8,11,14-15H,6,9-10,12H2,1-5H3,(H,21,26)/t14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 386 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446511

(CHEMBL3110096)Show InChI InChI=1S/C18H26N4O2/c1-4-12-23-15-6-7-16(14(3)13-15)21-8-10-22(11-9-21)18-19-17(5-2)24-20-18/h6-7,13H,4-5,8-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 391 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Mus musculus) | BDBM50446514

(CHEMBL3110076)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C2(CCC2)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C22H31N5O3/c1-5-29-18-7-8-19(15(2)13-18)26-11-12-27(16(3)14-26)21-23-20(30-25-21)22(9-6-10-22)24-17(4)28/h7-8,13,16H,5-6,9-12,14H2,1-4H3,(H,24,28)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ACC1 |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446529
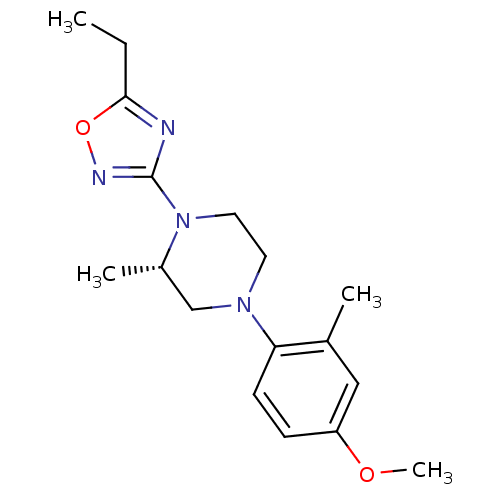
(CHEMBL3110080)Show SMILES CCc1nc(no1)N1CCN(C[C@@H]1C)c1ccc(OC)cc1C |r| Show InChI InChI=1S/C17H24N4O2/c1-5-16-18-17(19-23-16)21-9-8-20(11-13(21)3)15-7-6-14(22-4)10-12(15)2/h6-7,10,13H,5,8-9,11H2,1-4H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 486 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446530

(CHEMBL3110079)Show SMILES CCCOc1ccc(N2CCN(C(C2)C(F)(F)F)c2noc(CC)n2)c(C)c1 Show InChI InChI=1S/C19H25F3N4O2/c1-4-10-27-14-6-7-15(13(3)11-14)25-8-9-26(16(12-25)19(20,21)22)18-23-17(5-2)28-24-18/h6-7,11,16H,4-5,8-10,12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 492 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446524

(CHEMBL3110085)Show SMILES CCCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(Cl)c1 |r| Show InChI InChI=1S/C18H25ClN4O2/c1-4-10-24-14-6-7-16(15(19)11-14)22-8-9-23(13(3)12-22)18-20-17(5-2)25-21-18/h6-7,11,13H,4-5,8-10,12H2,1-3H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 688 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446516

(CHEMBL3110093)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)[C@@H](C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C20H29N5O3/c1-6-27-17-7-8-18(13(2)11-17)24-9-10-25(14(3)12-24)20-22-19(28-23-20)15(4)21-16(5)26/h7-8,11,14-15H,6,9-10,12H2,1-5H3,(H,21,26)/t14-,15+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 724 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446526
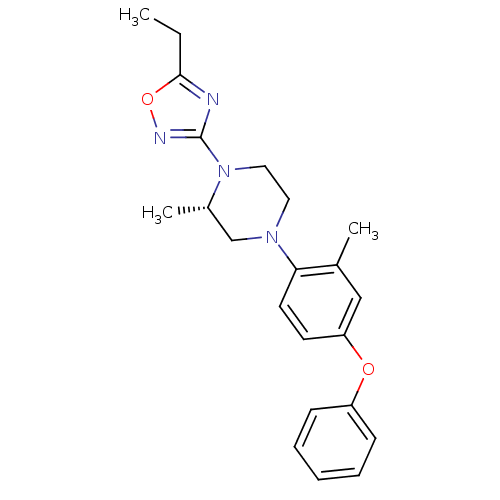
(CHEMBL3110083)Show SMILES CCc1nc(no1)N1CCN(C[C@@H]1C)c1ccc(Oc2ccccc2)cc1C |r| Show InChI InChI=1S/C22H26N4O2/c1-4-21-23-22(24-28-21)26-13-12-25(15-17(26)3)20-11-10-19(14-16(20)2)27-18-8-6-5-7-9-18/h5-11,14,17H,4,12-13,15H2,1-3H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 789 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446515

(CHEMBL3110075)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)C(C)(C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C21H31N5O3/c1-7-28-17-8-9-18(14(2)12-17)25-10-11-26(15(3)13-25)20-22-19(29-24-20)21(5,6)23-16(4)27/h8-9,12,15H,7,10-11,13H2,1-6H3,(H,23,27)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 811 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446513

(CHEMBL3110094)Show InChI InChI=1S/C19H20N2O2/c1-3-13-22-17-11-9-15(10-12-17)14-5-7-16(8-6-14)19-20-18(4-2)23-21-19/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 841 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446536

(CHEMBL3110097)Show SMILES CCCOc1ccc(N2CCN([C@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-11-24-16-7-8-17(14(3)12-16)22-9-10-23(15(4)13-22)19-20-18(6-2)25-21-19/h7-8,12,15H,5-6,9-11,13H2,1-4H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446512

(CHEMBL3110095)Show InChI InChI=1S/C20H22N2O2/c1-4-12-23-17-10-11-18(14(3)13-17)15-6-8-16(9-7-15)20-21-19(5-2)24-22-20/h6-11,13H,4-5,12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446525

(CHEMBL3110084)Show SMILES CCc1nc(no1)N1CCN(C[C@@H]1C)c1ccc(OC(F)(F)F)cc1C |r| Show InChI InChI=1S/C17H21F3N4O2/c1-4-15-21-16(22-26-15)24-8-7-23(10-12(24)3)14-6-5-13(9-11(14)2)25-17(18,19)20/h5-6,9,12H,4,7-8,10H2,1-3H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446535

(CHEMBL3110098)Show SMILES CCCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-11-24-16-7-8-17(14(3)12-16)22-9-10-23(15(4)13-22)19-20-18(6-2)25-21-19/h7-8,12,15H,5-6,9-11,13H2,1-4H3/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446534

(CHEMBL3110099)Show SMILES CCCOc1ccc(cc1)N1CCN([C@@H](C)C1)c1noc(CC)n1 |r| Show InChI InChI=1S/C18H26N4O2/c1-4-12-23-16-8-6-15(7-9-16)21-10-11-22(14(3)13-21)18-19-17(5-2)24-20-18/h6-9,14H,4-5,10-13H2,1-3H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446532

(CHEMBL3110077)Show SMILES CCCOc1ccc(N2CCN([C@@H](CC)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C20H30N4O2/c1-5-12-25-17-8-9-18(15(4)13-17)23-10-11-24(16(6-2)14-23)20-21-19(7-3)26-22-20/h8-9,13,16H,5-7,10-12,14H2,1-4H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446511

(CHEMBL3110096)Show InChI InChI=1S/C18H26N4O2/c1-4-12-23-15-6-7-16(14(3)13-15)21-8-10-22(11-9-21)18-19-17(5-2)24-20-18/h6-7,13H,4-5,8-12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446513

(CHEMBL3110094)Show InChI InChI=1S/C19H20N2O2/c1-3-13-22-17-11-9-15(10-12-17)14-5-7-16(8-6-14)19-20-18(4-2)23-21-19/h5-12H,3-4,13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446528

(CHEMBL3110081)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C18H26N4O2/c1-5-17-19-18(20-24-17)22-10-9-21(12-14(22)4)16-8-7-15(23-6-2)11-13(16)3/h7-8,11,14H,5-6,9-10,12H2,1-4H3/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50446521

(CHEMBL3110088)Show SMILES CCOc1cnc(N2CCN([C@@H](C)C2)c2noc(CC)n2)c(C)c1 |r| Show InChI InChI=1S/C17H25N5O2/c1-5-15-19-17(20-24-15)22-8-7-21(11-13(22)4)16-12(3)9-14(10-18-16)23-6-2/h9-10,13H,5-8,11H2,1-4H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 assessed as acetylCoA to malonylCoA conversion after 10 mins by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data