

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50197683 (CHEMBL3907479 | US10689705, Compound 49) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of FGFR2 (unknown origin) | ACS Med Chem Lett 8: 1264-1268 (2017) Article DOI: 10.1021/acsmedchemlett.7b00349 BindingDB Entry DOI: 10.7270/Q24170MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50197683 (CHEMBL3907479 | US10689705, Compound 49) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of FGFR1 (unknown origin) | ACS Med Chem Lett 8: 1264-1268 (2017) Article DOI: 10.1021/acsmedchemlett.7b00349 BindingDB Entry DOI: 10.7270/Q24170MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521804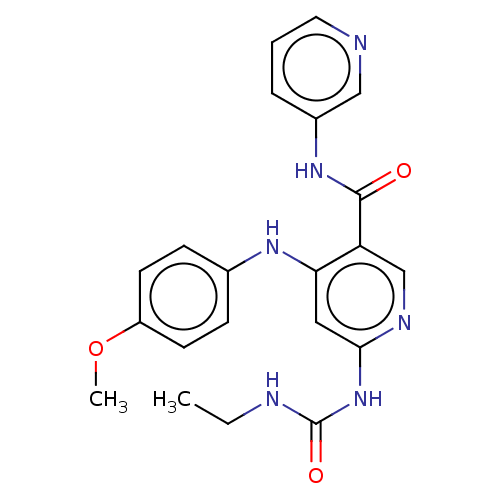 (CHEMBL4581035) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50197683 (CHEMBL3907479 | US10689705, Compound 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of FGFR3 (unknown origin) | ACS Med Chem Lett 8: 1264-1268 (2017) Article DOI: 10.1021/acsmedchemlett.7b00349 BindingDB Entry DOI: 10.7270/Q24170MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50315887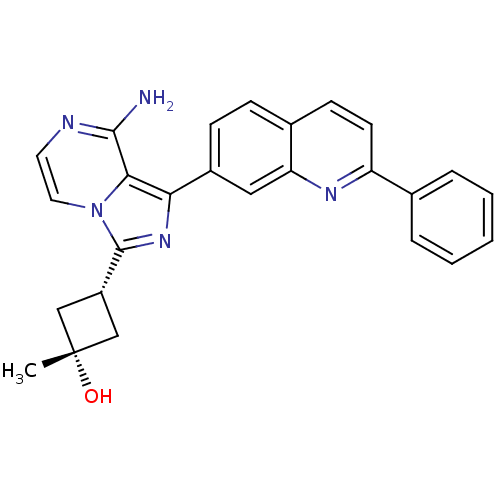 ((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Compound was tested for the biological activity at the Beta-1 adrenergic receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521806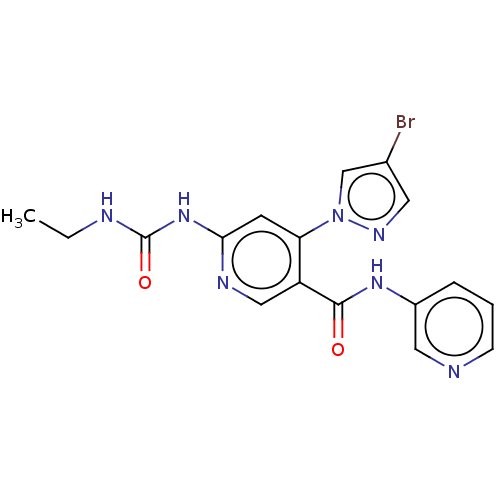 (CHEMBL4449908) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50315887 ((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Activity at beta-1 adrenergic receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521803 (CHEMBL4568624) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521799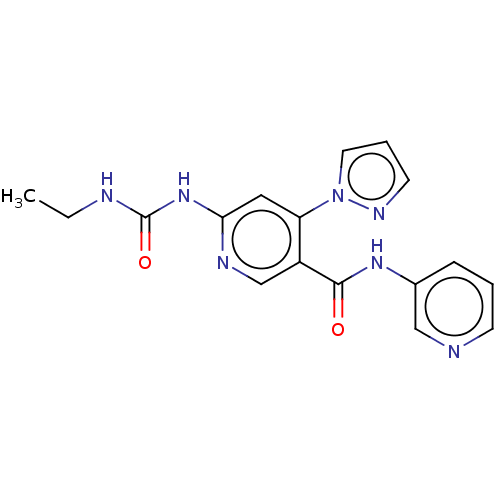 (CHEMBL4459270) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50157879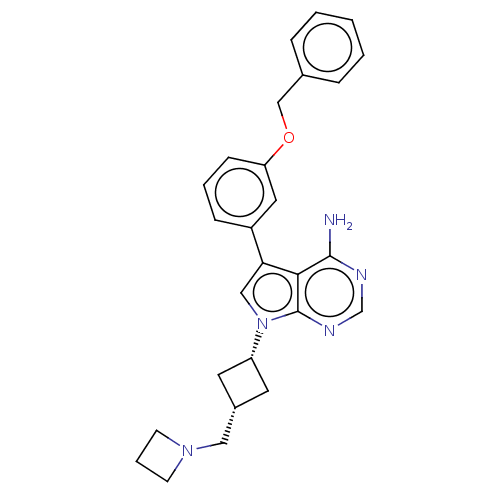 (CHEMBL1614712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Activity at beta-1 adrenergic receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521800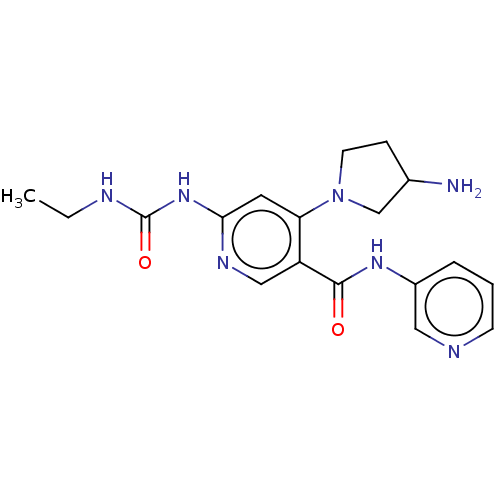 (CHEMBL4470688) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50582143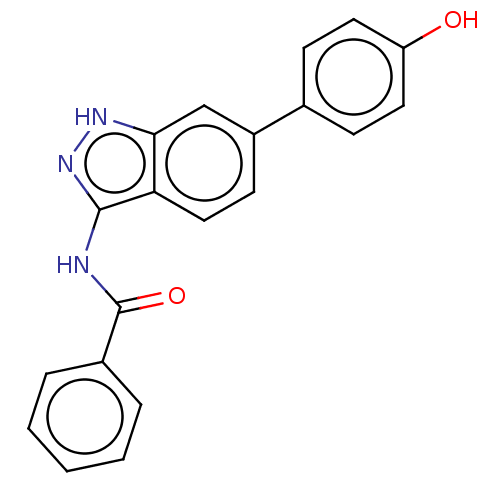 (CHEMBL4095682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type FGFR2 (unknown origin) (461 to 763 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521805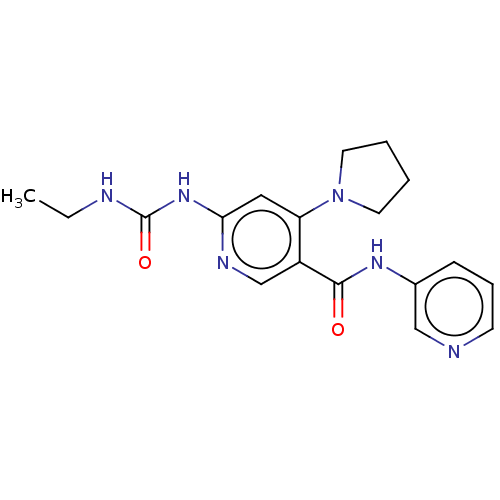 (CHEMBL4436644) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50582148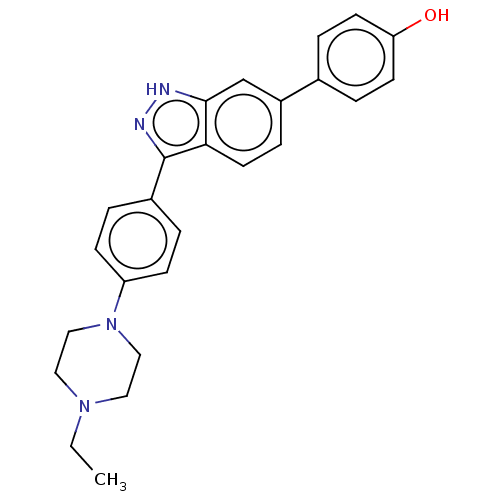 (CHEMBL5077959) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50582142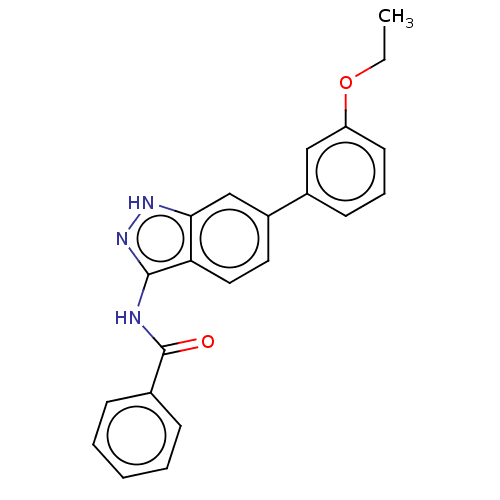 (CHEMBL5088013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type FGFR2 (unknown origin) (461 to 763 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521801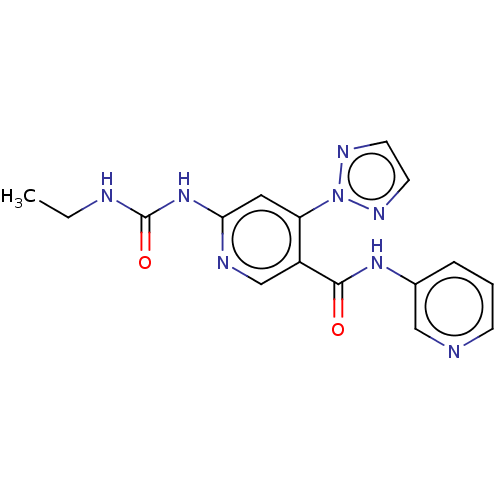 (CHEMBL4521306) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521802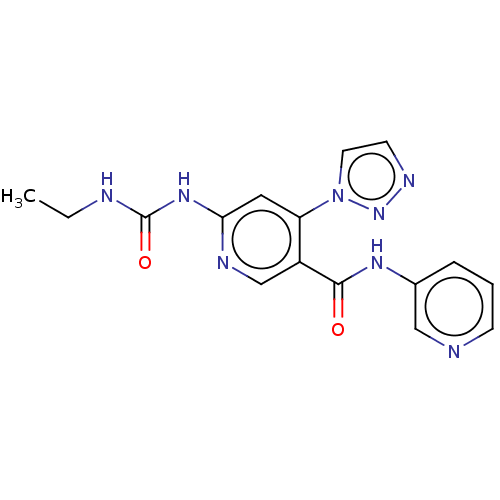 (CHEMBL4465300) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50582146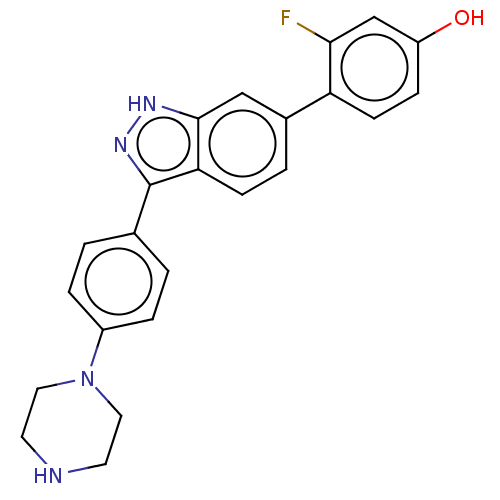 (CHEMBL5085478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50339906 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Escherichia coli B ER2560 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leuco... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50339906 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Escherichia coli B ER2560 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leuco... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine--tRNA ligase (Staphylococcus aureus (strain NCTC 8325 / PS 47)) | BDBM50339906 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Staphylococcus aureus seg50 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leu... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine--tRNA ligase (Staphylococcus aureus (strain NCTC 8325 / PS 47)) | BDBM50339906 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Staphylococcus aureus seg50 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leu... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50582150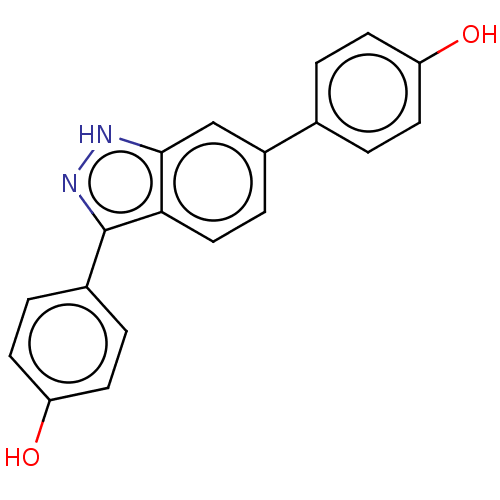 (CHEMBL5091513) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type FGFR2 (unknown origin) (461 to 763 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Staphylococcus aureus (strain NCTC 8325 / PS 47)) | BDBM50530866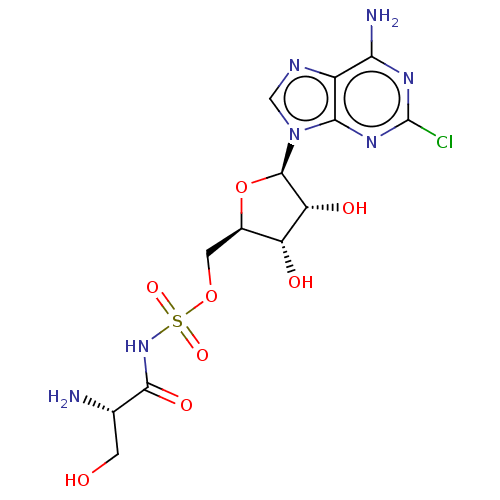 (CHEMBL4529844) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Staphylococcus aureus seg50 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leu... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Staphylococcus aureus (strain NCTC 8325 / PS 47)) | BDBM50530866 (CHEMBL4529844) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Staphylococcus aureus seg50 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leu... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50582147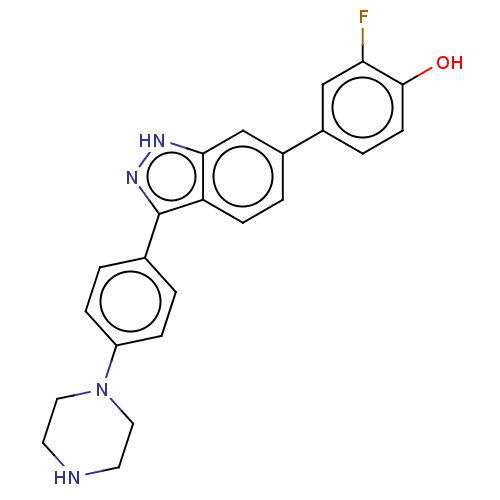 (CHEMBL5083349) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50589513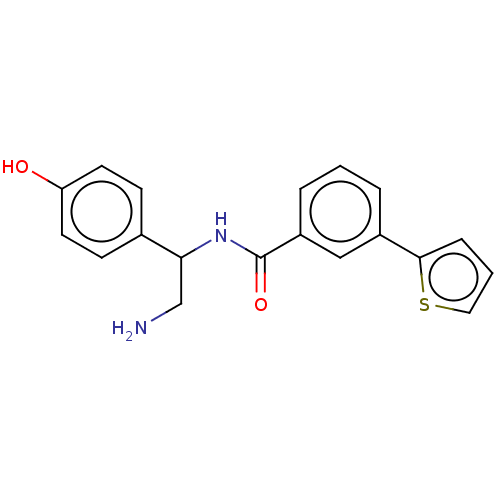 (CHEMBL5188460) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00049k BindingDB Entry DOI: 10.7270/Q2R2159M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Staphylococcus aureus (strain NCTC 8325 / PS 47)) | BDBM50530864 (CHEMBL4467050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Staphylococcus aureus seg50 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leu... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Staphylococcus aureus (strain NCTC 8325 / PS 47)) | BDBM50530864 (CHEMBL4467050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Staphylococcus aureus seg50 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leu... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50582144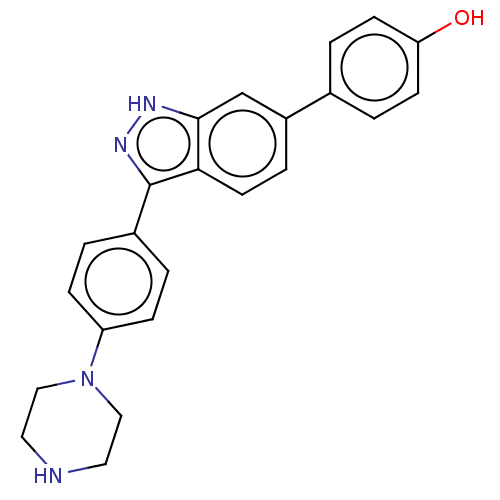 (CHEMBL5090566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50582143 (CHEMBL4095682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM50521807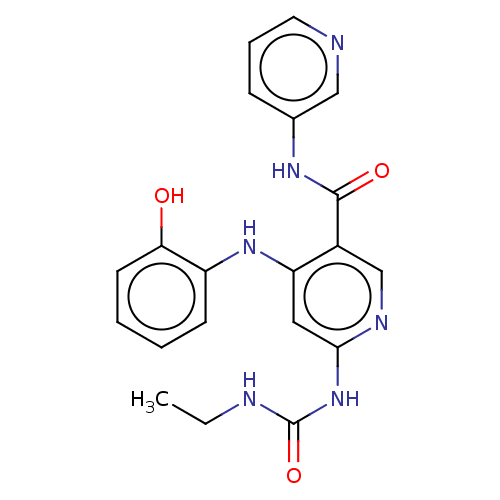 (CHEMBL4577939) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase(A2B2 tetramer) preincubated for 5 mins followed by ATP addition and measured once per minute for 20 to 30 m... | Bioorg Med Chem 27: 3546-3550 (2019) Article DOI: 10.1016/j.bmc.2019.06.015 BindingDB Entry DOI: 10.7270/Q2NK3JFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50582145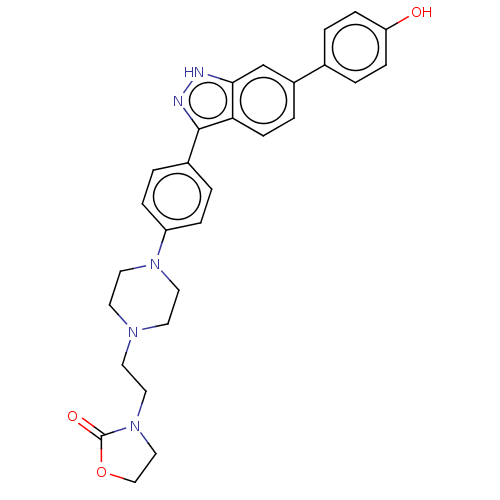 (CHEMBL5084070) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 449 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50530866 (CHEMBL4529844) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Escherichia coli B ER2560 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leuco... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50530866 (CHEMBL4529844) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Escherichia coli B ER2560 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leuco... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50582142 (CHEMBL5088013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00049k BindingDB Entry DOI: 10.7270/Q2R2159M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50280100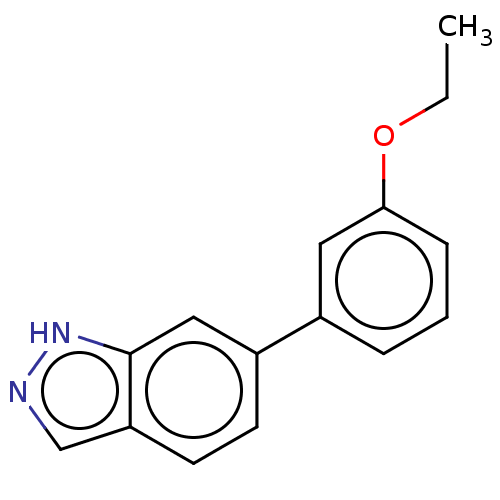 (CHEMBL4172184) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged FGFR2 cytoplasmic domain (403 to 822 residues) expressed in baculovirus expression system using Tyr 04 as ... | ACS Med Chem Lett 8: 1264-1268 (2017) Article DOI: 10.1021/acsmedchemlett.7b00349 BindingDB Entry DOI: 10.7270/Q24170MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50280100 (CHEMBL4172184) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type FGFR2 (unknown origin) (461 to 763 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50582141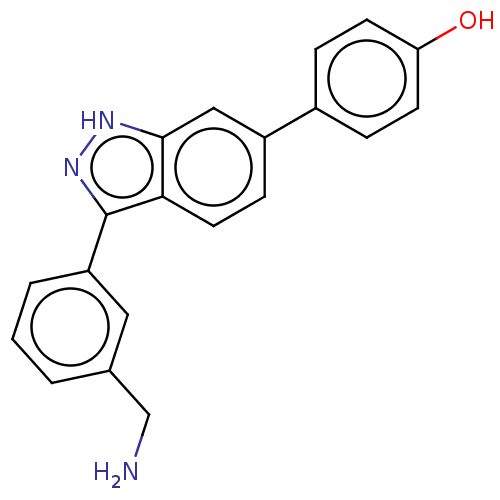 (CHEMBL5075056) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type FGFR2 (unknown origin) (461 to 763 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Staphylococcus aureus (strain NCTC 8325 / PS 47)) | BDBM50530862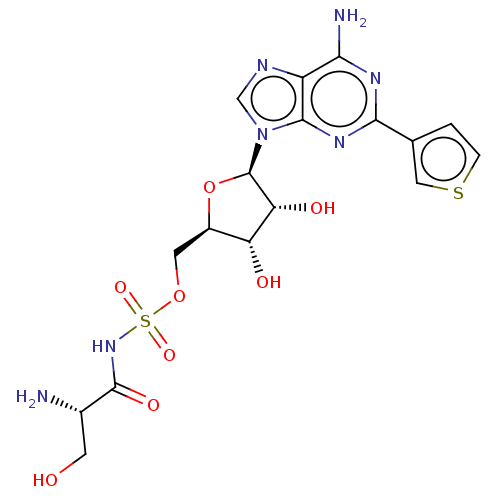 (CHEMBL4446951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Staphylococcus aureus seg50 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leu... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Staphylococcus aureus (strain NCTC 8325 / PS 47)) | BDBM50530862 (CHEMBL4446951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Staphylococcus aureus seg50 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leu... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50530864 (CHEMBL4467050) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Escherichia coli B ER2560 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leuco... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50530864 (CHEMBL4467050) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Escherichia coli B ER2560 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leuco... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50530862 (CHEMBL4446951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Escherichia coli B ER2560 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leuco... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine--tRNA ligase (Escherichia coli (strain K12)) | BDBM50530862 (CHEMBL4446951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of C-terminal His10-tagged Escherichia coli B ER2560 SerRS expressed in Lemo21(DE3) using Ap4A as substrate in presence of serine by leuco... | J Med Chem 62: 9703-9717 (2019) Article DOI: 10.1021/acs.jmedchem.9b01131 BindingDB Entry DOI: 10.7270/Q2GX4G11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50280100 (CHEMBL4172184) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged FGFR1 cytoplasmic domain (308 to 731 residues) expressed in baculovirus expression system using Tyr 04 as ... | ACS Med Chem Lett 8: 1264-1268 (2017) Article DOI: 10.1021/acsmedchemlett.7b00349 BindingDB Entry DOI: 10.7270/Q24170MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50280100 (CHEMBL4172184) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50582139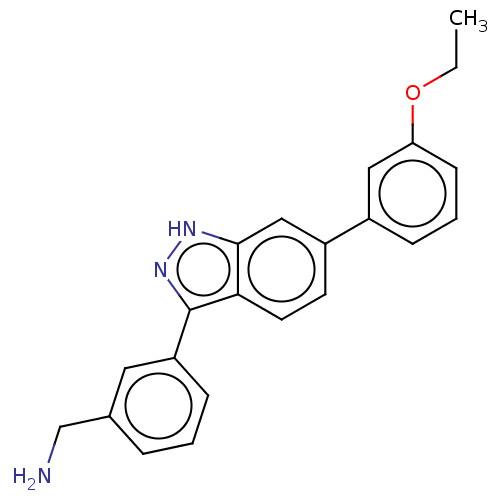 (CHEMBL5069932) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type FGFR2 (unknown origin) (461 to 763 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50582150 (CHEMBL5091513) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FGFR1 (unknown origin) (458 to 765 residues) transfected in Escherichia coli BL21(DE3) cells using Tyr04 peptide as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01163 BindingDB Entry DOI: 10.7270/Q2DB85Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 194 total ) | Next | Last >> |