 Found 30 hits with Last Name = 'fisk' and Initial = 'ha'
Found 30 hits with Last Name = 'fisk' and Initial = 'ha' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.184 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 using GEEEEYFELVKKKK as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.351 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human TYK2 using KKSRGDYMTMQIG as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.542 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human PKCa using histone H1 as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human SYK using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human PKACA using LCGRTGRRNSI as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human YES/YES1 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human LCK using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human PKCg using histone H1 as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.82 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.94 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 using KGSGSGRPRTSSFAEG as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human ZAP70 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human FAK/PTK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20.3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclinD1 using RB-CTF as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45.8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human MEK1 using ERK2 as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human ABL1 using EAIYAAPFAKKK as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR/FRAP1 using 4EBP1 as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50264841

(CHEMBL4076767)Show SMILES COc1cc(ccc1Nc1nc(NC2CCCCC2)c2cc[nH]c2n1)N1CCOCC1 Show InChI InChI=1S/C23H30N6O2/c1-30-20-15-17(29-11-13-31-14-12-29)7-8-19(20)26-23-27-21-18(9-10-24-21)22(28-23)25-16-5-3-2-4-6-16/h7-10,15-16H,2-6,11-14H2,1H3,(H3,24,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 356 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged Mps1/TTK (507 to 1400 residues) using recombinant cetn2 as substrate in presence of [gamma-32P]ATP measure... |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 581 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1 using ATF2 as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50053315
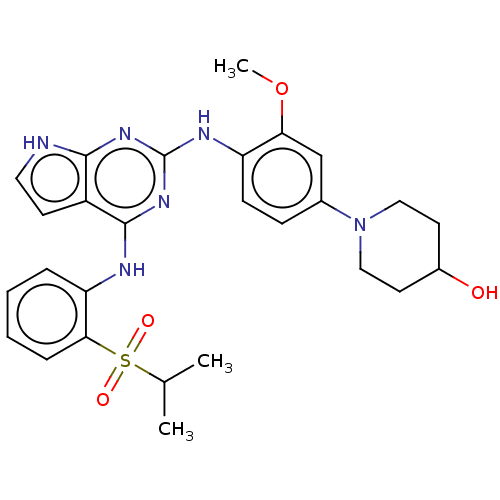
(CHEMBL3330411)Show SMILES COc1cc(ccc1Nc1nc(Nc2ccccc2S(=O)(=O)C(C)C)c2cc[nH]c2n1)N1CCC(O)CC1 Show InChI InChI=1S/C27H32N6O4S/c1-17(2)38(35,36)24-7-5-4-6-22(24)29-26-20-10-13-28-25(20)31-27(32-26)30-21-9-8-18(16-23(21)37-3)33-14-11-19(34)12-15-33/h4-10,13,16-17,19,34H,11-12,14-15H2,1-3H3,(H3,28,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 809 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged Mps1/TTK (507 to 1400 residues) using recombinant cetn2 as substrate in presence of [gamma-32P]ATP measure... |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50264841

(CHEMBL4076767)Show SMILES COc1cc(ccc1Nc1nc(NC2CCCCC2)c2cc[nH]c2n1)N1CCOCC1 Show InChI InChI=1S/C23H30N6O2/c1-30-20-15-17(29-11-13-31-14-12-29)7-8-19(20)26-23-27-21-18(9-10-24-21)22(28-23)25-16-5-3-2-4-6-16/h7-10,15-16H,2-6,11-14H2,1H3,(H3,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 891 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human FAK/PTK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50264841

(CHEMBL4076767)Show SMILES COc1cc(ccc1Nc1nc(NC2CCCCC2)c2cc[nH]c2n1)N1CCOCC1 Show InChI InChI=1S/C23H30N6O2/c1-30-20-15-17(29-11-13-31-14-12-29)7-8-19(20)26-23-27-21-18(9-10-24-21)22(28-23)25-16-5-3-2-4-6-16/h7-10,15-16H,2-6,11-14H2,1H3,(H3,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1 using ATF2 as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM36485

(1-(4-(4-(2-(Isopropylsulfonyl)phenylamino)-1H-pyrr...)Show SMILES COc1cc(ccc1Nc1cc(Nc2ccccc2S(=O)(=O)C(C)C)c2cc[nH]c2n1)N1CCC(O)CC1 Show InChI InChI=1S/C28H33N5O4S/c1-18(2)38(35,36)26-7-5-4-6-23(26)30-24-17-27(32-28-21(24)10-13-29-28)31-22-9-8-19(16-25(22)37-3)33-14-11-20(34)12-15-33/h4-10,13,16-18,20,34H,11-12,14-15H2,1-3H3,(H3,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged Mps1/TTK (507 to 1400 residues) using recombinant cetn2 as substrate in presence of [gamma-32P]ATP measure... |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50264842

(CHEMBL4061742)Show SMILES COc1ccccc1Nc1nc(Nc2ccccc2S(=O)(=O)C(C)C)c2cc[nH]c2n1 Show InChI InChI=1S/C22H23N5O3S/c1-14(2)31(28,29)19-11-7-5-9-17(19)24-21-15-12-13-23-20(15)26-22(27-21)25-16-8-4-6-10-18(16)30-3/h4-14H,1-3H3,(H3,23,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged Mps1/TTK (507 to 1400 residues) using recombinant cetn2 as substrate in presence of [gamma-32P]ATP measure... |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50264843
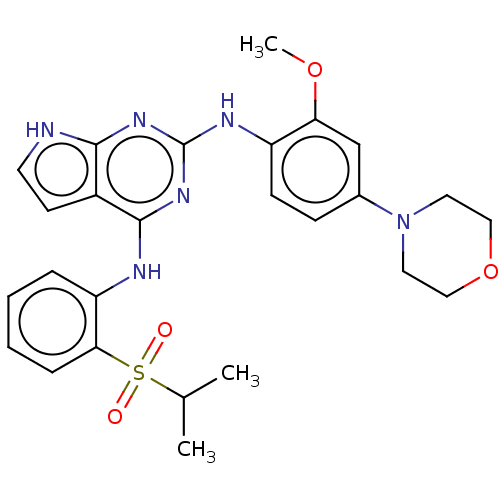
(CHEMBL4091309)Show SMILES COc1cc(ccc1Nc1nc(Nc2ccccc2S(=O)(=O)C(C)C)c2cc[nH]c2n1)N1CCOCC1 Show InChI InChI=1S/C26H30N6O4S/c1-17(2)37(33,34)23-7-5-4-6-21(23)28-25-19-10-11-27-24(19)30-26(31-25)29-20-9-8-18(16-22(20)35-3)32-12-14-36-15-13-32/h4-11,16-17H,12-15H2,1-3H3,(H3,27,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged Mps1/TTK (507 to 1400 residues) using recombinant cetn2 as substrate in presence of [gamma-32P]ATP measure... |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50264841

(CHEMBL4076767)Show SMILES COc1cc(ccc1Nc1nc(NC2CCCCC2)c2cc[nH]c2n1)N1CCOCC1 Show InChI InChI=1S/C23H30N6O2/c1-30-20-15-17(29-11-13-31-14-12-29)7-8-19(20)26-23-27-21-18(9-10-24-21)22(28-23)25-16-5-3-2-4-6-16/h7-10,15-16H,2-6,11-14H2,1H3,(H3,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50264841

(CHEMBL4076767)Show SMILES COc1cc(ccc1Nc1nc(NC2CCCCC2)c2cc[nH]c2n1)N1CCOCC1 Show InChI InChI=1S/C23H30N6O2/c1-30-20-15-17(29-11-13-31-14-12-29)7-8-19(20)26-23-27-21-18(9-10-24-21)22(28-23)25-16-5-3-2-4-6-16/h7-10,15-16H,2-6,11-14H2,1H3,(H3,24,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human TYK2 using KKSRGDYMTMQIG as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50264841

(CHEMBL4076767)Show SMILES COc1cc(ccc1Nc1nc(NC2CCCCC2)c2cc[nH]c2n1)N1CCOCC1 Show InChI InChI=1S/C23H30N6O2/c1-30-20-15-17(29-11-13-31-14-12-29)7-8-19(20)26-23-27-21-18(9-10-24-21)22(28-23)25-16-5-3-2-4-6-16/h7-10,15-16H,2-6,11-14H2,1H3,(H3,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human SYK using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data