

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50396066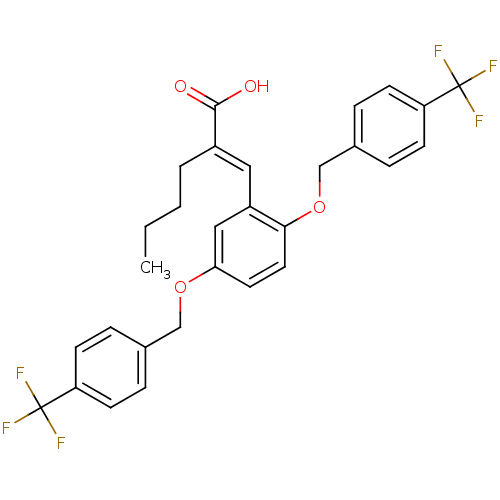 (CHEMBL1819479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062724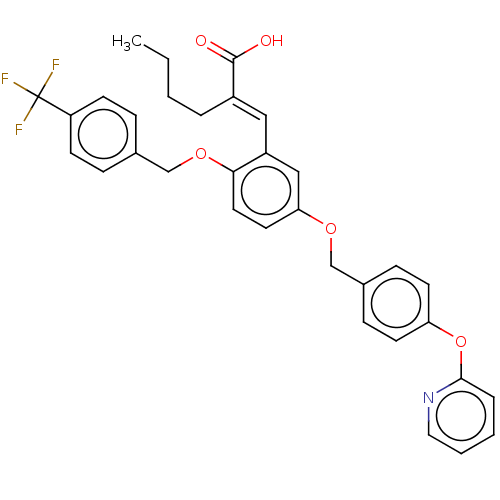 (CHEMBL3397723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062727 (CHEMBL3397721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062722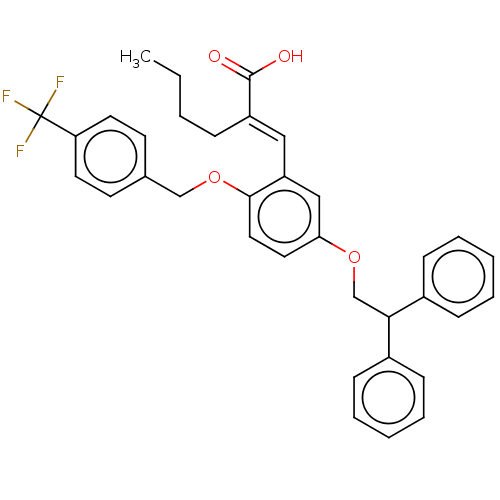 (CHEMBL3397726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062730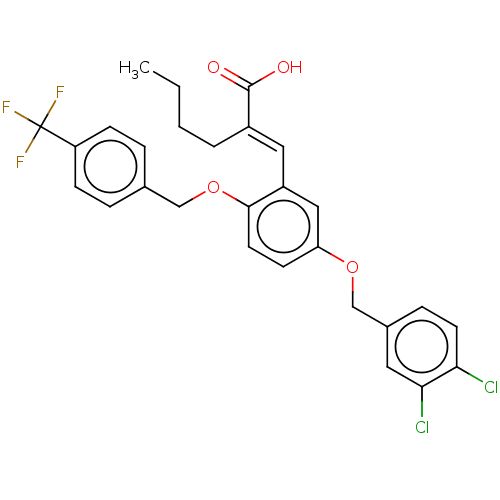 (CHEMBL3397716) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062731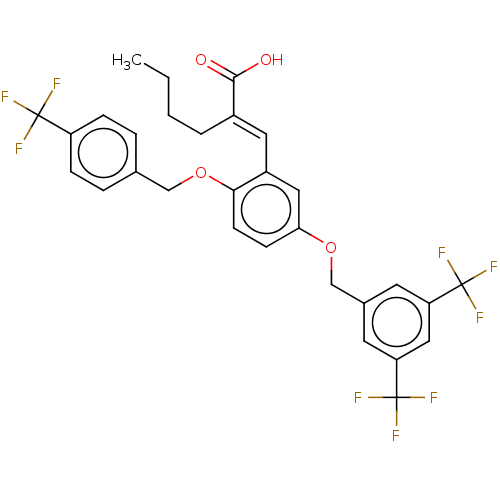 (CHEMBL3397717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062723 (CHEMBL3397728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062729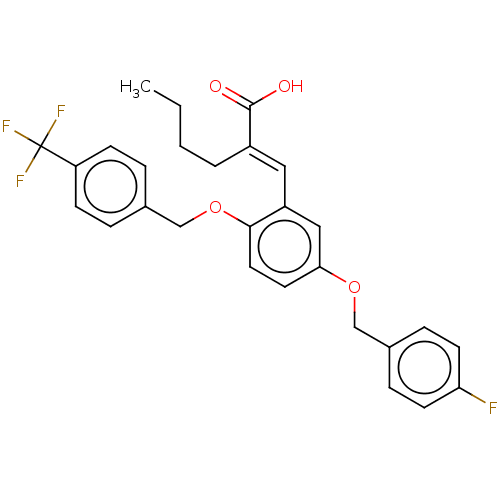 (CHEMBL3397715) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062621 (CHEMBL3397710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50273683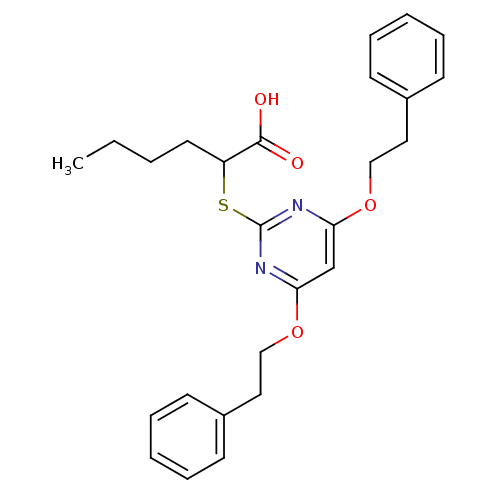 (2-(4,6-Diphenethoxypyrimidin-2-ylthio)hexanoic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062727 (CHEMBL3397721) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062733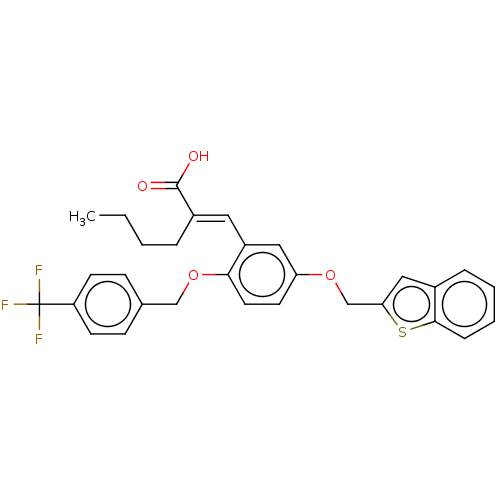 (CHEMBL3397719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062718 (CHEMBL3397711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062731 (CHEMBL3397717) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50322460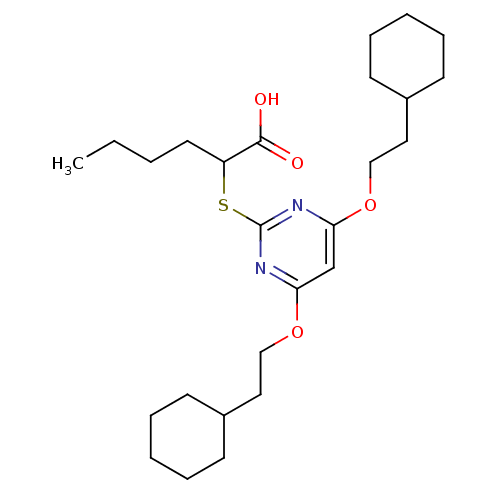 (2-(4,6-Bis(2-cyclohexylethoxy)pyrimidin-2-ylthio)h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062728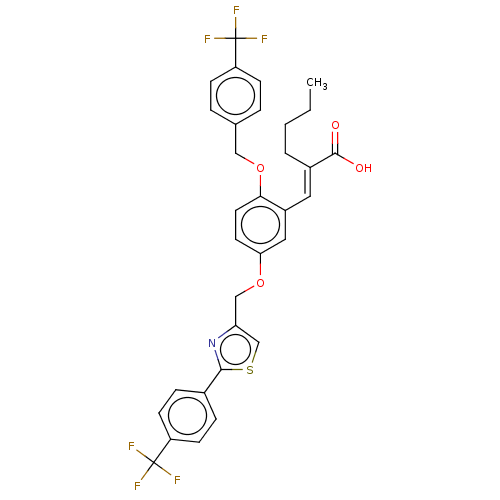 (CHEMBL3397720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062721 (CHEMBL3397724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062726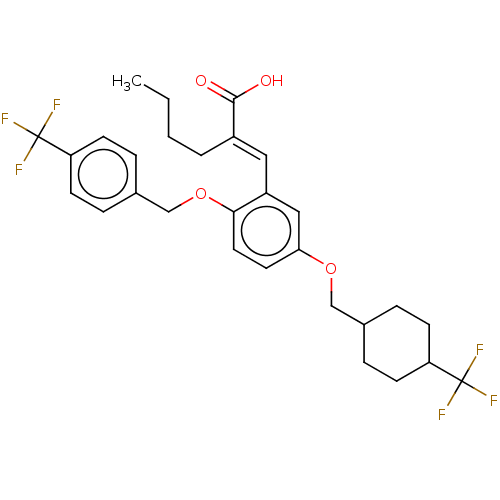 (CHEMBL3397727) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50396066 (CHEMBL1819479) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062723 (CHEMBL3397728) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062730 (CHEMBL3397716) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062726 (CHEMBL3397727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062730 (CHEMBL3397716) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062719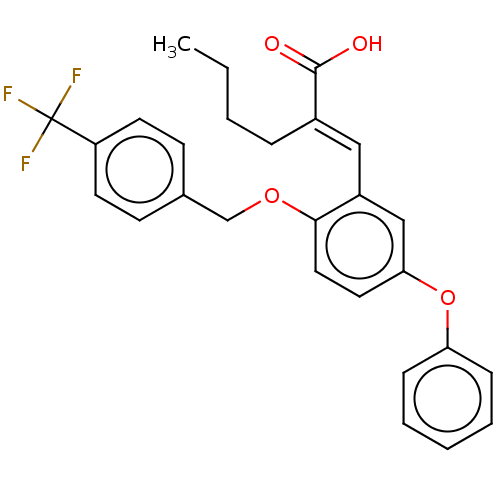 (CHEMBL3397709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062721 (CHEMBL3397724) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062728 (CHEMBL3397720) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062732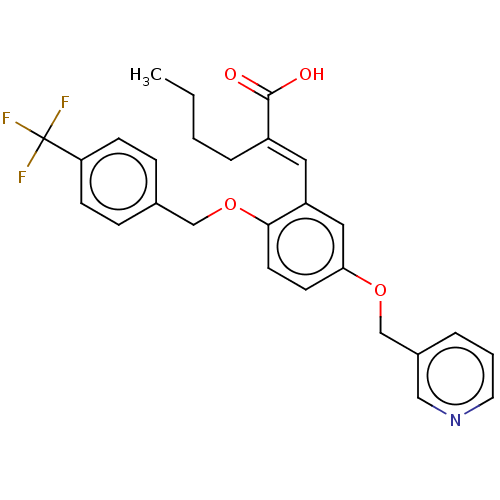 (CHEMBL3397718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062737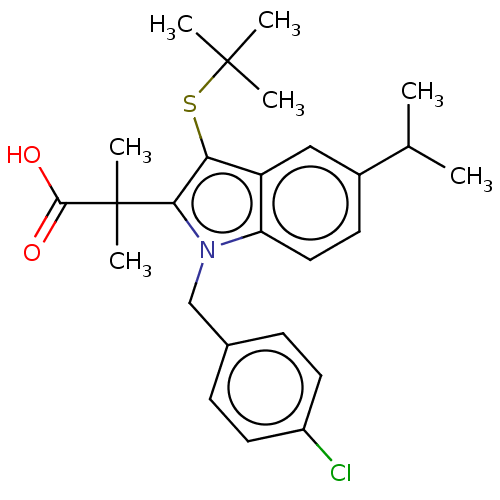 (CHEMBL3397729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062726 (CHEMBL3397727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062727 (CHEMBL3397721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062723 (CHEMBL3397728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062718 (CHEMBL3397711) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062733 (CHEMBL3397719) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062736 (CHEMBL3397712) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062729 (CHEMBL3397715) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062718 (CHEMBL3397711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062724 (CHEMBL3397723) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50322460 (2-(4,6-Bis(2-cyclohexylethoxy)pyrimidin-2-ylthio)h...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062621 (CHEMBL3397710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062720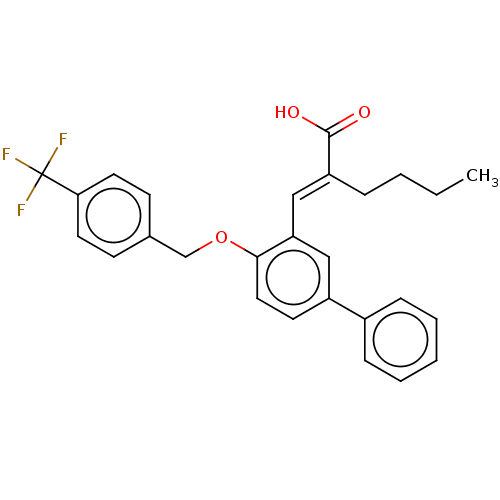 (CHEMBL3397708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50062725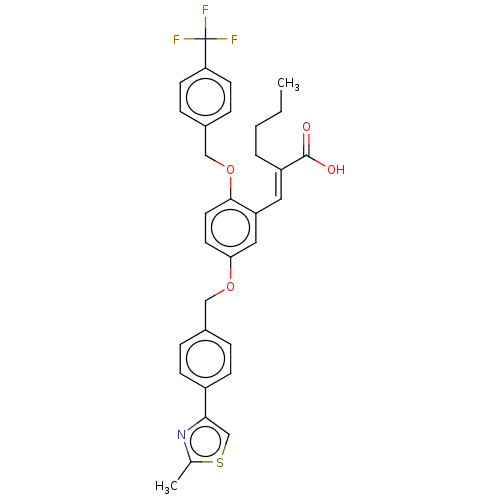 (CHEMBL3397722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062729 (CHEMBL3397715) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50062734 (CHEMBL3397714) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of wild-type human presenilin-1 stably overexpressed in CHO cells co-expressing wild-type human amyloid precursor protein assessed as inhi... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062721 (CHEMBL3397724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50062733 (CHEMBL3397719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in interleukin-1beta-stimulated human A549 microsomal membranes preincubated for 15 mins before PGH2 ad... | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50396066 (CHEMBL1819479) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of ovine COX1-mediated 12-HHT formation preincubated for 5 mins before arachidonic acid addition by HPLC analysis | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50062621 (CHEMBL3397710) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of ovine COX1-mediated 12-HHT formation preincubated for 5 mins before arachidonic acid addition by HPLC analysis | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50062718 (CHEMBL3397711) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of ovine COX1-mediated 12-HHT formation preincubated for 5 mins before arachidonic acid addition by HPLC analysis | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50062729 (CHEMBL3397715) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of ovine COX1-mediated 12-HHT formation preincubated for 5 mins before arachidonic acid addition by HPLC analysis | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50062730 (CHEMBL3397716) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of ovine COX1-mediated 12-HHT formation preincubated for 5 mins before arachidonic acid addition by HPLC analysis | Bioorg Med Chem Lett 25: 841-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.073 BindingDB Entry DOI: 10.7270/Q2280989 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 304 total ) | Next | Last >> |