

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apelin receptor (Homo sapiens (Human)) | BDBM50583259 (CHEMBL5094438) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479471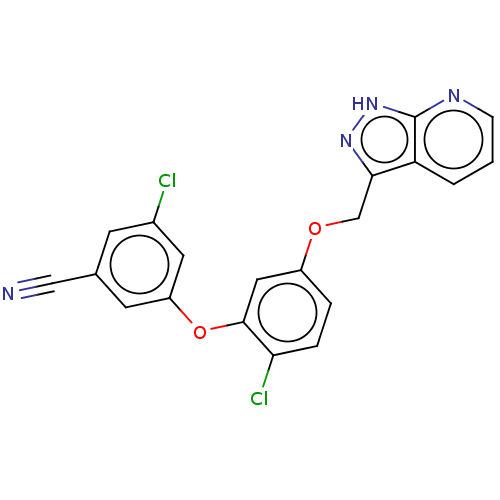 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484029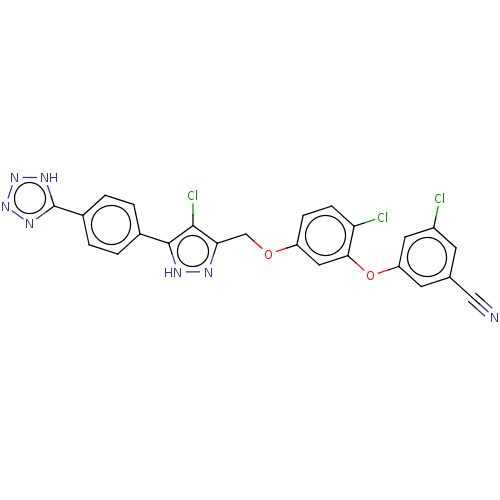 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484039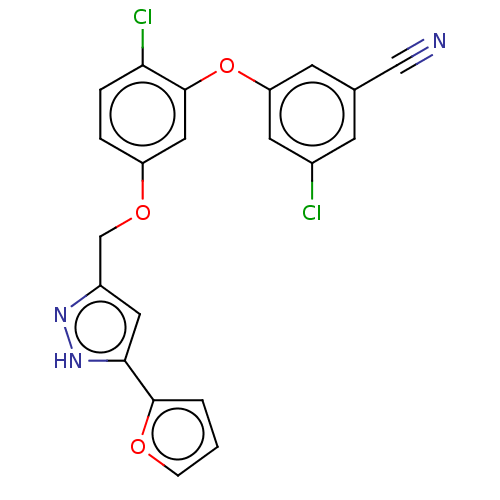 (CHEMBL1800087) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484635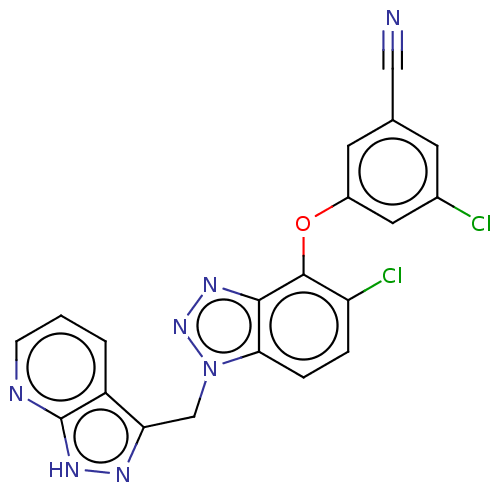 (CHEMBL1939500) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484045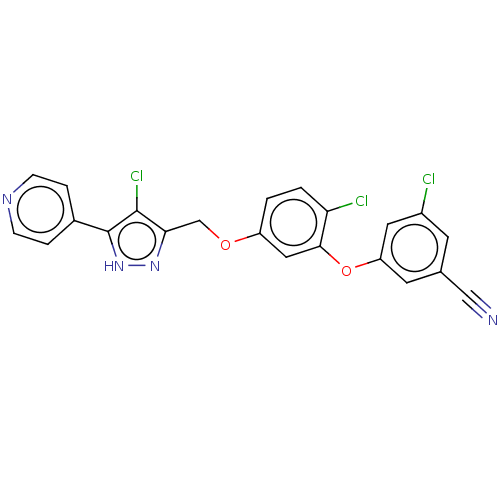 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484045 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484632 (Mk-6186 | Mk6186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50583256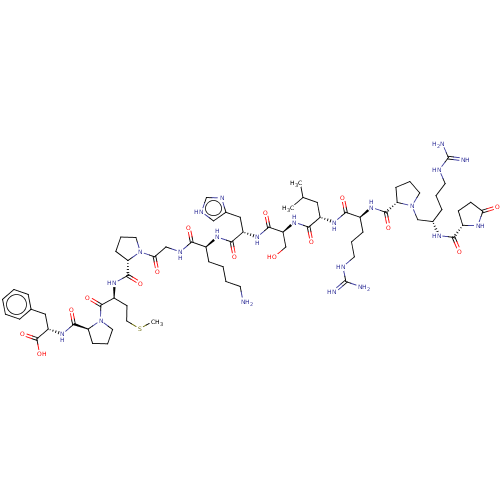 (CHEMBL5087847) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50583266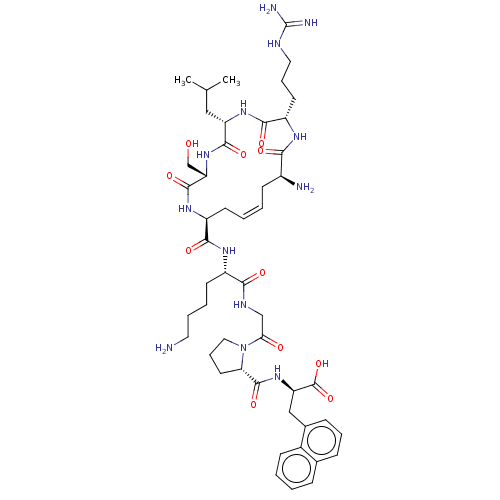 (CHEMBL5091501) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484029 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484029 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484032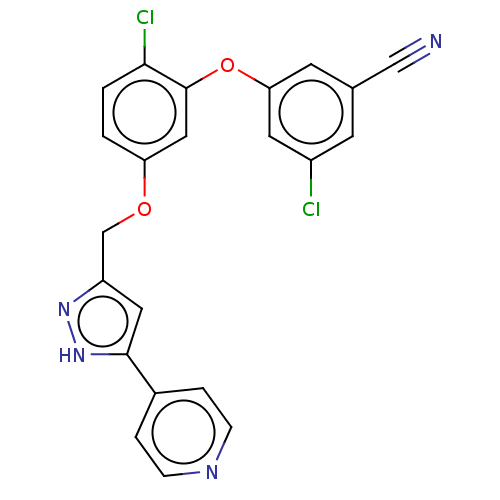 (CHEMBL1801231) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484031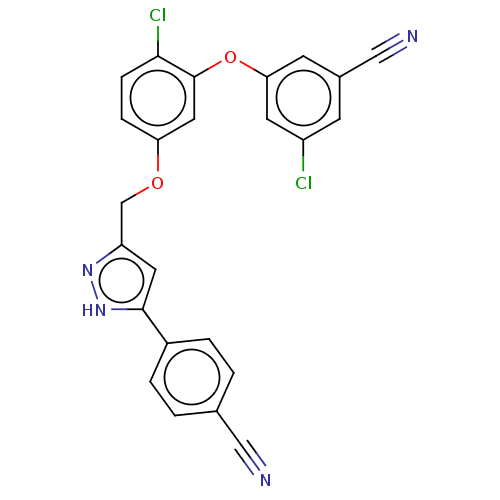 (CHEMBL1801255) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484040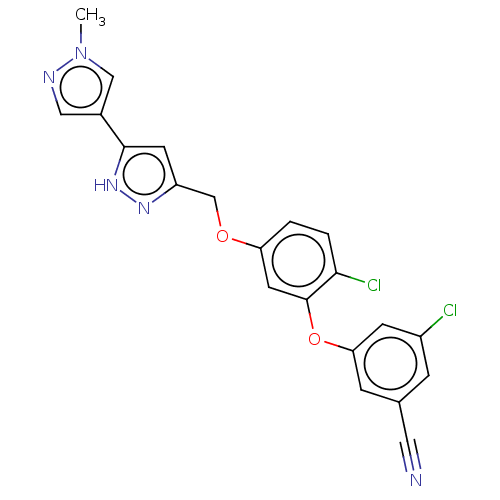 (CHEMBL1801228) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484045 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484022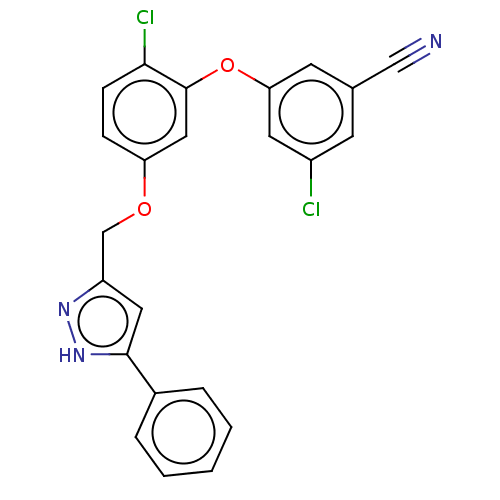 (CHEMBL1801223) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50583258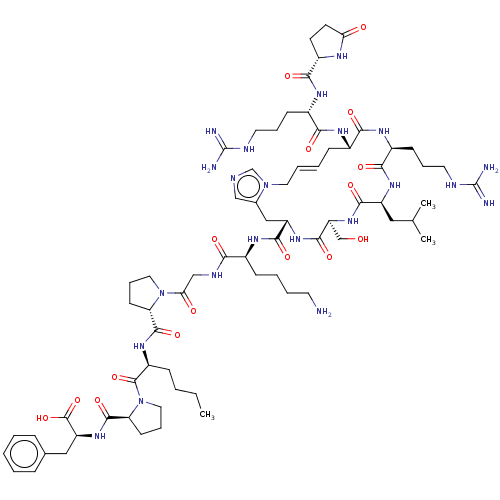 (CHEMBL5078149) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484629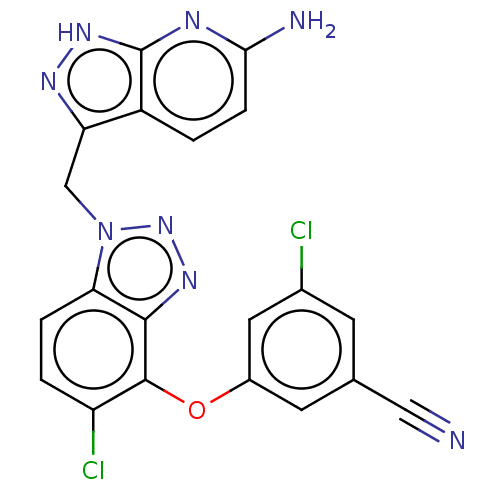 (CHEMBL1939503) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484023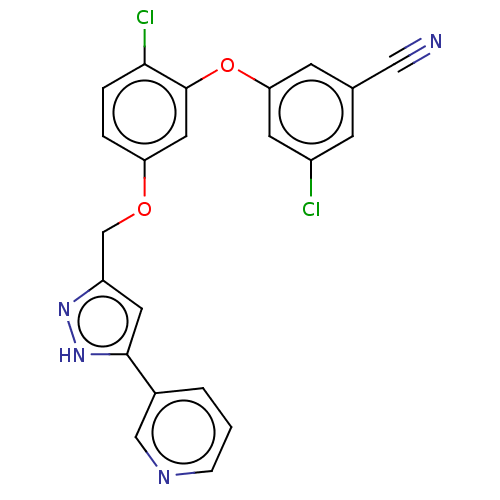 (CHEMBL1801230) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484039 (CHEMBL1800087) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484024 (CHEMBL1801227) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50583261 (CHEMBL5092962) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484630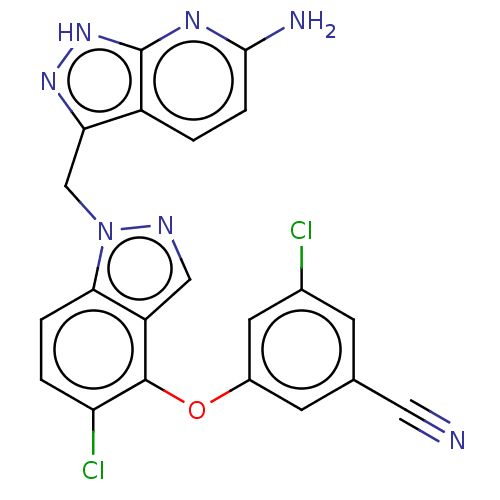 (CHEMBL1939502 | MK-7445) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50583263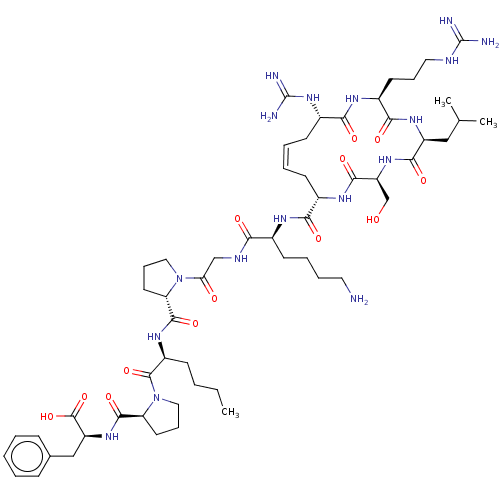 (CHEMBL5091081) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484035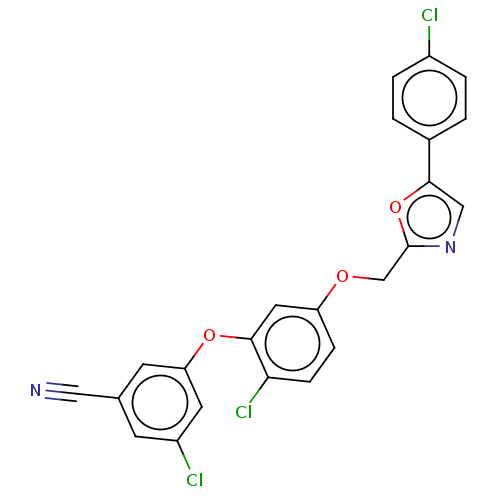 (CHEMBL1801266) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484043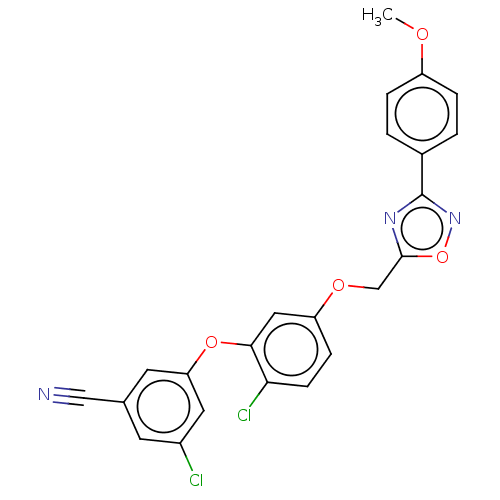 (CHEMBL1801262) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484022 (CHEMBL1801223) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50583260 (CHEMBL5072626) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484026 (CHEMBL1801225) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484040 (CHEMBL1801228) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50244534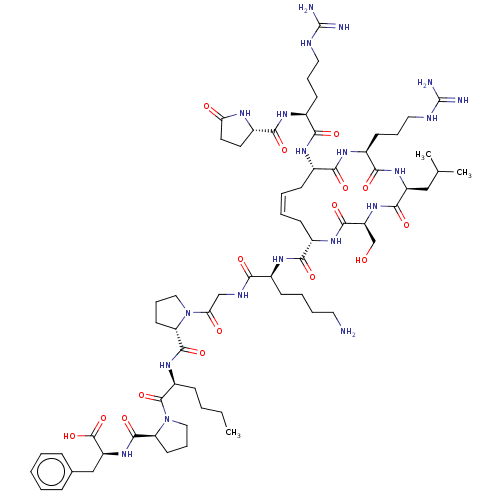 (CHEMBL4083079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484033 (CHEMBL1801229) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484031 (CHEMBL1801255) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484624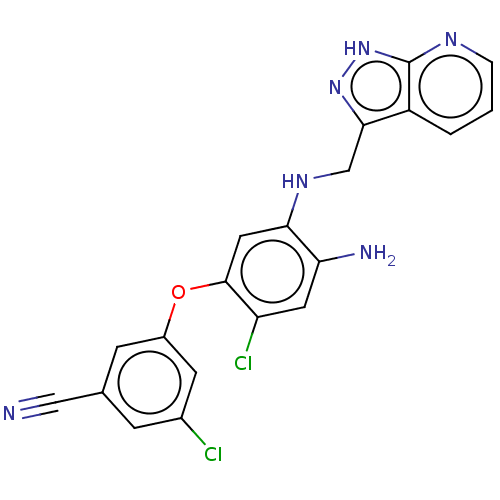 (CHEMBL1939510) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484032 (CHEMBL1801231) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484040 (CHEMBL1801228) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484631 (CHEMBL1939501) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50583257 (CHEMBL5080649) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484035 (CHEMBL1801266) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484024 (CHEMBL1801227) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50583262 (CHEMBL5079665) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-[Nle75,Tyr77][Pyr1]-Ape13 from YFP-tagged human APJ expressed in HEK293 cell membranes assessed as inhibition constant incubat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01708 BindingDB Entry DOI: 10.7270/Q20R9T9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484032 (CHEMBL1801231) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484036 (CHEMBL1801265) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 638 total ) | Next | Last >> |