

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423171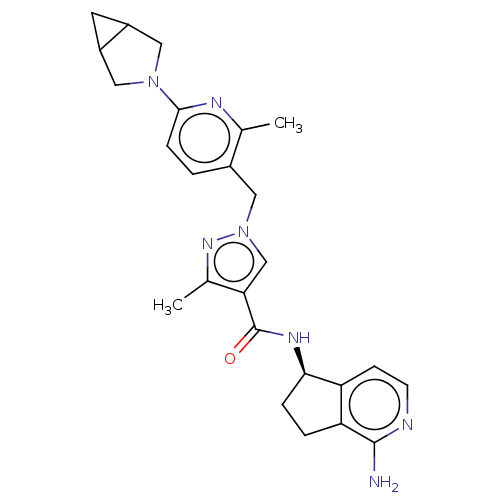 (US10501440, Example 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423183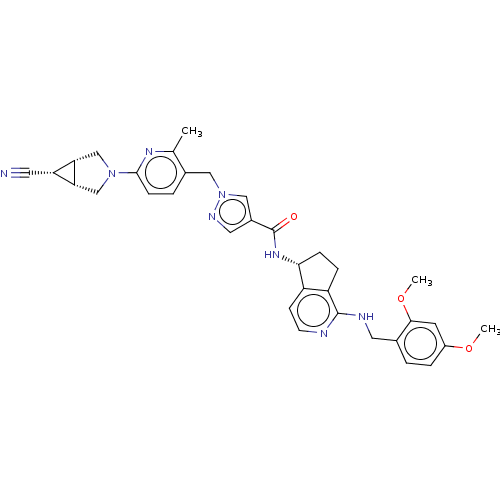 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423170 (US10501440, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423169 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423179 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423174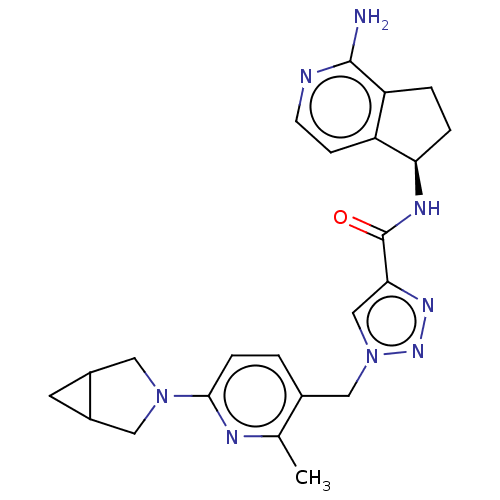 (US10501440, Example 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423173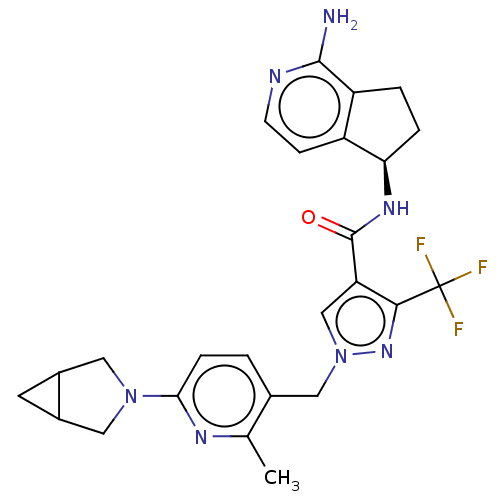 (US10501440, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423180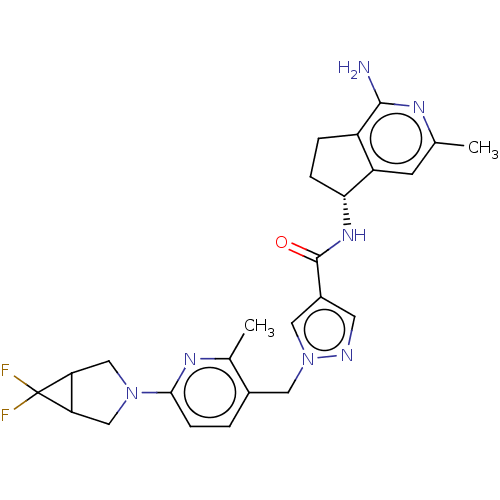 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423185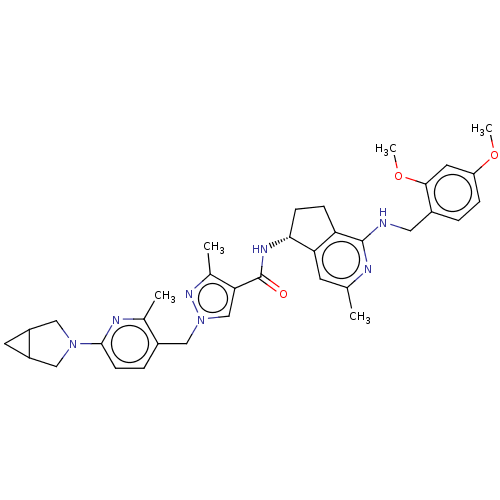 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423187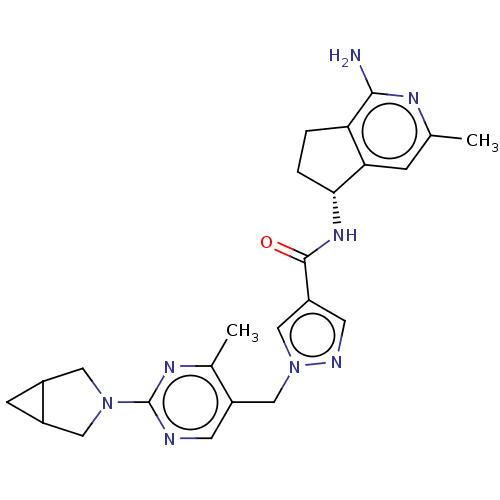 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423188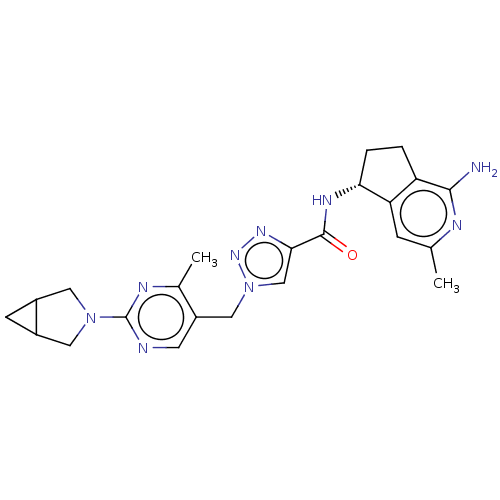 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423182 (US10501440, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423184 (US10501440, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423181 (US10501440, Example 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423176 (US10501440, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423186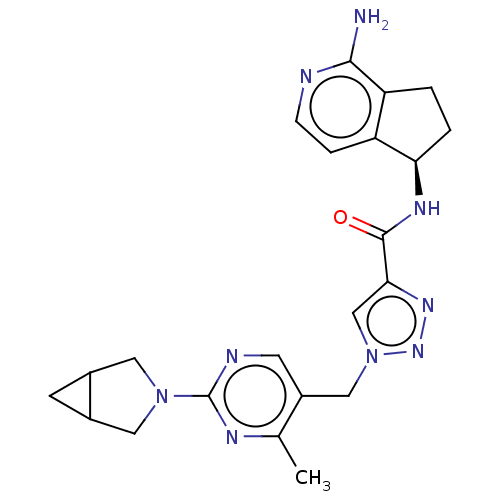 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM171943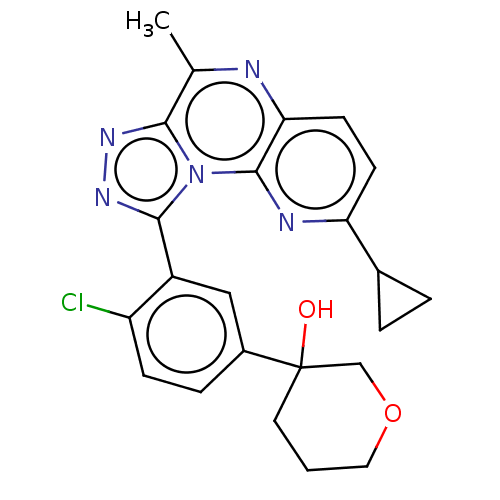 (US9085584, 113) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US9085584 (2015) BindingDB Entry DOI: 10.7270/Q27P8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423170 (US10501440, Example 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423180 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423169 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423185 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423183 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423179 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423195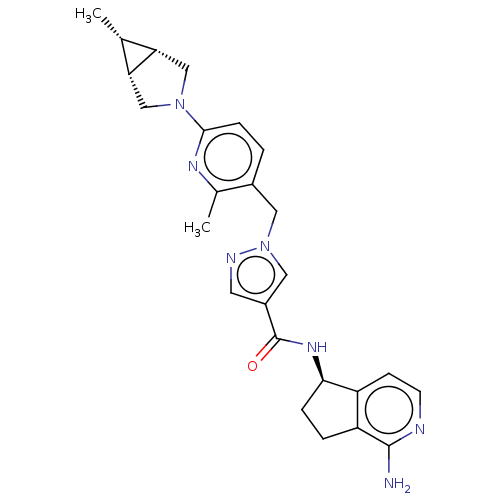 (1-[2-Methyl-6-((1S,5R,6R)-6-methyl-3-aza-bicyclo[3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423171 (US10501440, Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423173 (US10501440, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423174 (US10501440, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM171929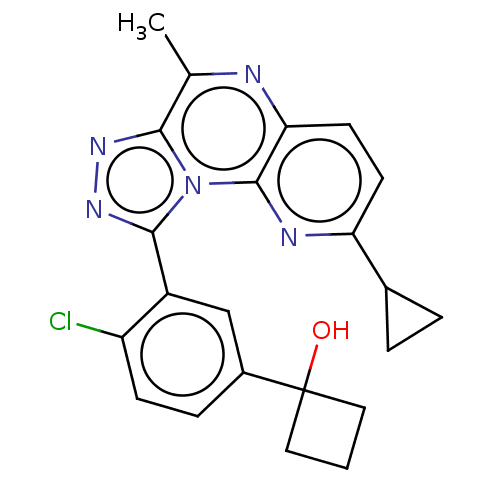 (US9085584, 88) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US9085584 (2015) BindingDB Entry DOI: 10.7270/Q27P8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM171932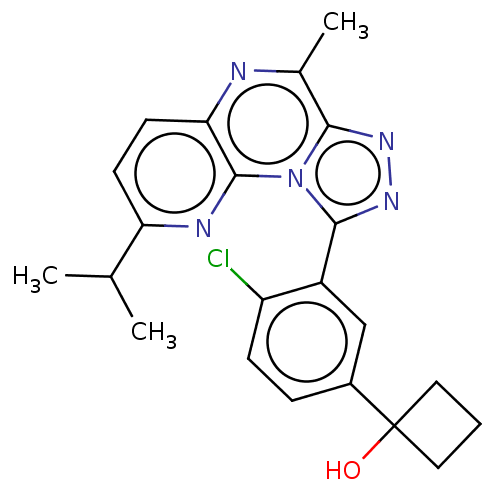 (US9085584, 91) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US9085584 (2015) BindingDB Entry DOI: 10.7270/Q27P8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441037 (US10640486, Example 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441028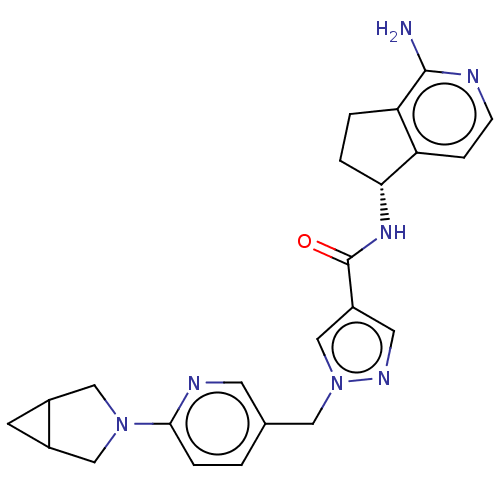 (1-[6-(3-Aza-bicyclo[3.1.0]hex-3-yl)-pyridin-3-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441047 (US10640486, Example 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441010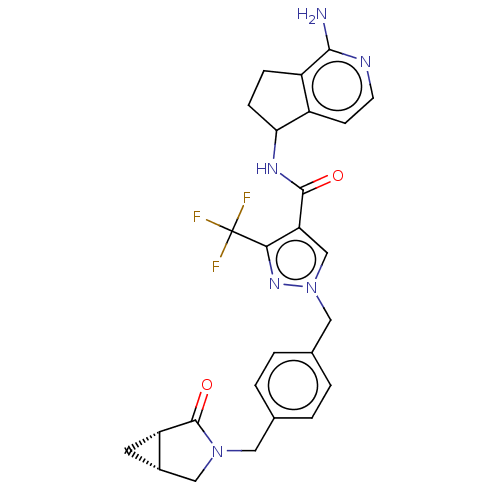 (US10640486, Example 50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441002 (US10640486, Example 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM441001 (US10640486, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM440998 (US10640486, Example 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM440996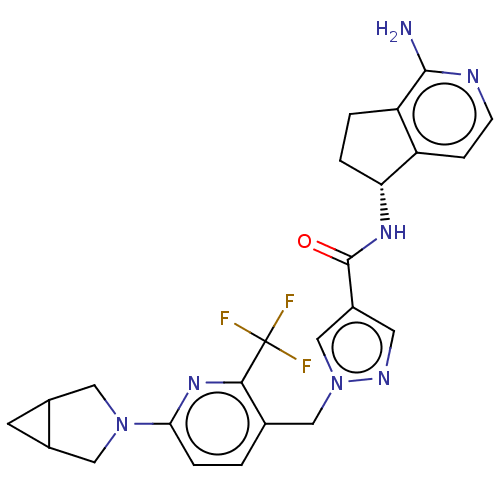 (US10640486, Example 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640486 (2020) BindingDB Entry DOI: 10.7270/Q2X92F98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423189 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423187 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423175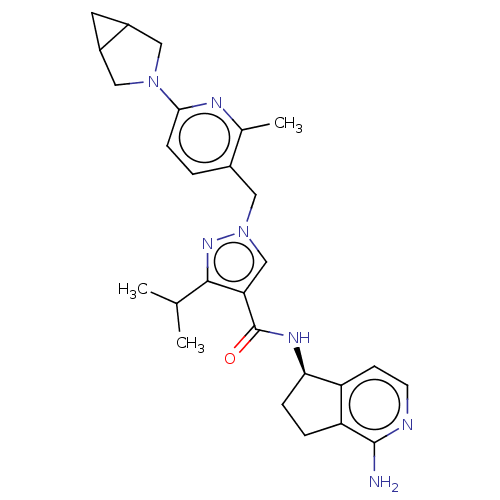 (US10501440, Example 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM412370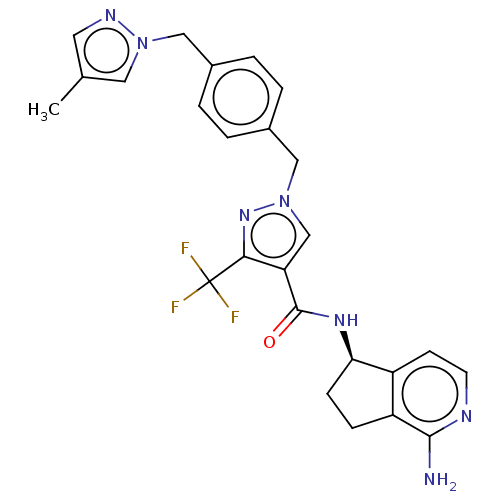 (US10399961, Example 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 hr at Room Temperature with 0.10... | US Patent US10399961 (2019) BindingDB Entry DOI: 10.7270/Q22Z17X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM171910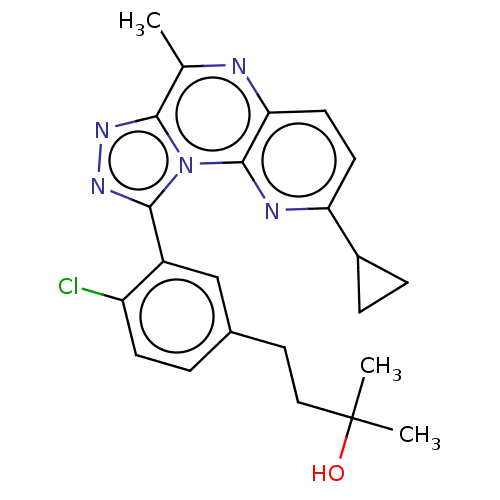 (US9085584, 69) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US9085584 (2015) BindingDB Entry DOI: 10.7270/Q27P8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM171890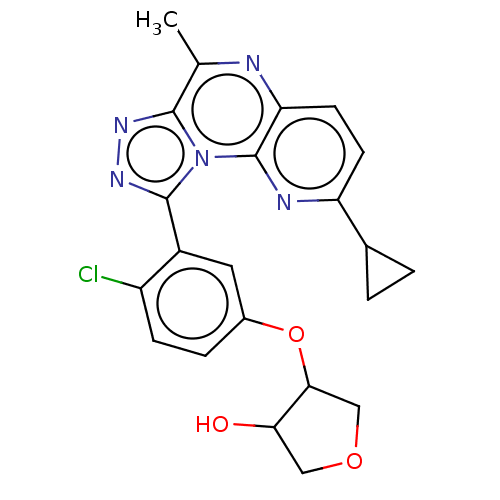 (US9085584, 56 | US9085584, 56a | US9085584, 56b | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US9085584 (2015) BindingDB Entry DOI: 10.7270/Q27P8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM171935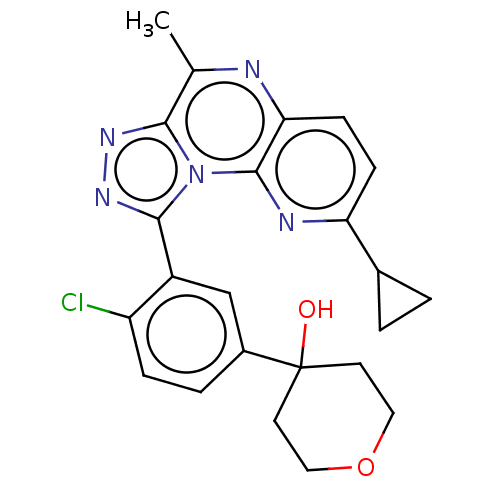 (US9085584, 94) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US9085584 (2015) BindingDB Entry DOI: 10.7270/Q27P8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM171839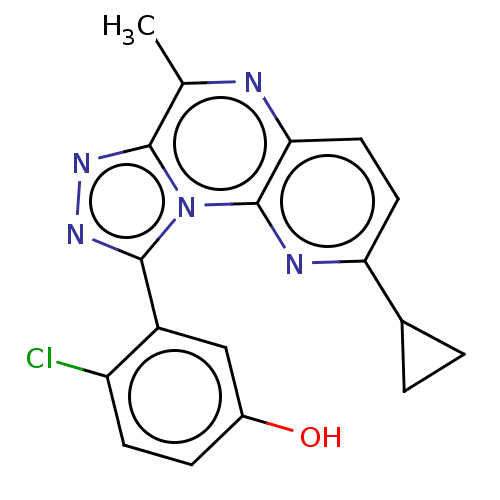 (US9085584, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US9085584 (2015) BindingDB Entry DOI: 10.7270/Q27P8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423193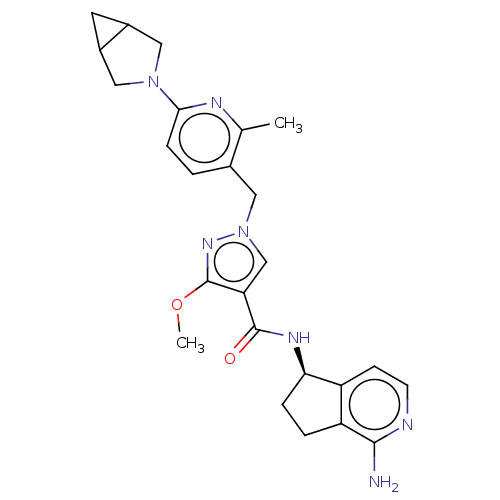 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423176 (US10501440, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM284757 (US10023575, Example 48 | US10023575, Example 49 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10479794 (2019) BindingDB Entry DOI: 10.7270/Q27083TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM476570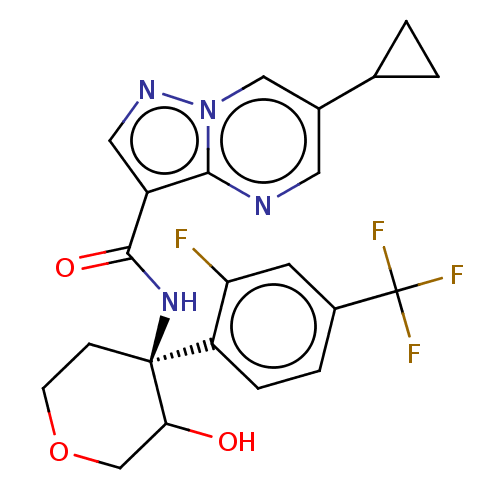 (US10875867, Example 82a | US11691977, Example 82b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... | US Patent US10875867 (2020) BindingDB Entry DOI: 10.7270/Q2WQ06W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423197 (1-[6-(5-Aza-spiro[2.3]hex-5-yl)-2-methyl-pyridin-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1372 total ) | Next | Last >> |