

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50441620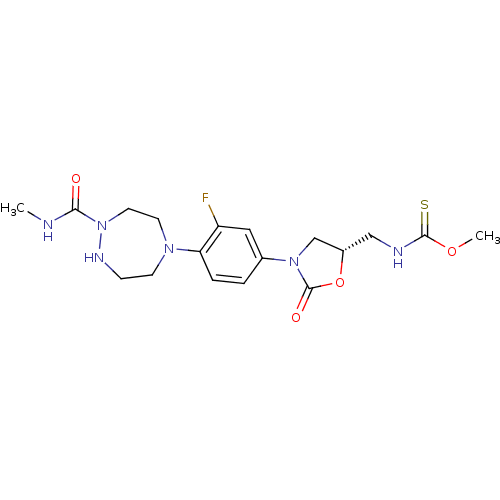 (CHEMBL2437199) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 69: 262-77 (2013) Article DOI: 10.1016/j.ejmech.2013.08.002 BindingDB Entry DOI: 10.7270/Q2DJ5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50116067 ((Linezolid)N-[3-(3-Fluoro-4-morpholin-4-yl-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 assessed as ethoxyresorufin O-deethylation after 20 mins by fluorescence plate reader analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441620 (CHEMBL2437199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes assessed as tolbutamide hydroxylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 69: 262-77 (2013) Article DOI: 10.1016/j.ejmech.2013.08.002 BindingDB Entry DOI: 10.7270/Q2DJ5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441621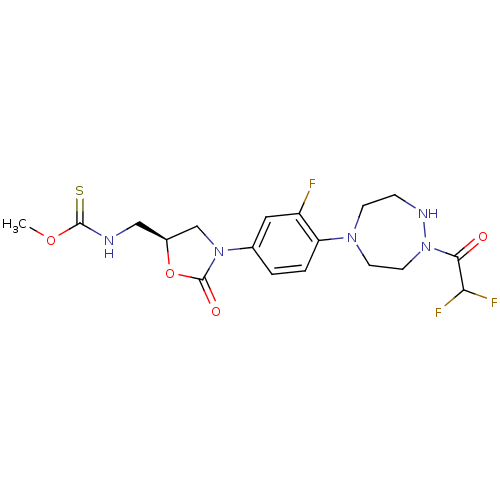 (CHEMBL2437330) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes assessed as tolbutamide hydroxylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 69: 262-77 (2013) Article DOI: 10.1016/j.ejmech.2013.08.002 BindingDB Entry DOI: 10.7270/Q2DJ5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50441620 (CHEMBL2437199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes assessed as ethoxyresorufin O-deethylation after 20 mins by fluorescence plate reader analysis | Eur J Med Chem 69: 262-77 (2013) Article DOI: 10.1016/j.ejmech.2013.08.002 BindingDB Entry DOI: 10.7270/Q2DJ5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50441621 (CHEMBL2437330) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes assessed as ethoxyresorufin O-deethylation after 20 mins by fluorescence plate reader analysis | Eur J Med Chem 69: 262-77 (2013) Article DOI: 10.1016/j.ejmech.2013.08.002 BindingDB Entry DOI: 10.7270/Q2DJ5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50433366 (CHEMBL2377663) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 assessed as dextromethorphan O-demethylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50433367 (CHEMBL2377662) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 assessed as dextromethorphan O-demethylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50433368 (EPEREZOLID | U-100592) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 assessed as dextromethorphan O-demethylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50116067 ((Linezolid)N-[3-(3-Fluoro-4-morpholin-4-yl-phenyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 assessed as dextromethorphan O-demethylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50433366 (CHEMBL2377663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 assessed as tolbutamide hydroxylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50433367 (CHEMBL2377662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 assessed as tolbutamide hydroxylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50433368 (EPEREZOLID | U-100592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 assessed as tolbutamide hydroxylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50116067 ((Linezolid)N-[3-(3-Fluoro-4-morpholin-4-yl-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 assessed as tolbutamide hydroxylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50433366 (CHEMBL2377663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 assessed as ethoxyresorufin O-deethylation after 20 mins by fluorescence plate reader analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50433367 (CHEMBL2377662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 assessed as ethoxyresorufin O-deethylation after 20 mins by fluorescence plate reader analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50433368 (EPEREZOLID | U-100592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 assessed as ethoxyresorufin O-deethylation after 20 mins by fluorescence plate reader analysis | Eur J Med Chem 63: 811-25 (2013) Article DOI: 10.1016/j.ejmech.2013.03.003 BindingDB Entry DOI: 10.7270/Q29K4CMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50441621 (CHEMBL2437330) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Foundation Itsuu Laboratory Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 20 mins by LC/MS/MS analysis | Eur J Med Chem 69: 262-77 (2013) Article DOI: 10.1016/j.ejmech.2013.08.002 BindingDB Entry DOI: 10.7270/Q2DJ5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||