

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arylamine N-acetyltransferase (Mycobacterium smegmatis) | BDBM50336284 (3-[3'-(2''cyclopent-2'''-en-1'''-ylphenoxy)-2'-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis NAT using isoniazed substrate by acetyl-coA hydrolysis assay | Bioorg Med Chem Lett 21: 1185-90 (2011) Article DOI: 10.1016/j.bmcl.2010.12.099 BindingDB Entry DOI: 10.7270/Q2SJ1KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylamine N-acetyltransferase (Pseudomonas aeruginosa) | BDBM50336284 (3-[3'-(2''cyclopent-2'''-en-1'''-ylphenoxy)-2'-hyd...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa NAT using 5-aminosalicylate substrate by acetyl-coA hydrolysis assay | Bioorg Med Chem Lett 21: 1185-90 (2011) Article DOI: 10.1016/j.bmcl.2010.12.099 BindingDB Entry DOI: 10.7270/Q2SJ1KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylamine N-acetyltransferase (Mycobacterium smegmatis) | BDBM50336284 (3-[3'-(2''cyclopent-2'''-en-1'''-ylphenoxy)-2'-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis NAT using 5-aminosalicylate substrate by acetyl-coA hydrolysis assay | Bioorg Med Chem Lett 21: 1185-90 (2011) Article DOI: 10.1016/j.bmcl.2010.12.099 BindingDB Entry DOI: 10.7270/Q2SJ1KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylamine N-acetyltransferase (Pseudomonas aeruginosa) | BDBM50336284 (3-[3'-(2''cyclopent-2'''-en-1'''-ylphenoxy)-2'-hyd...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa NAT using isoniazed substrate by acetyl-coA hydrolysis assay | Bioorg Med Chem Lett 21: 1185-90 (2011) Article DOI: 10.1016/j.bmcl.2010.12.099 BindingDB Entry DOI: 10.7270/Q2SJ1KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylamine N-acetyltransferase (Pseudomonas aeruginosa) | BDBM50336283 (3-[3'-(4-cyclopent-2'''-en-1'''-ylphenoxy)-2'-hydr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa NAT using isoniazed substrate by acetyl-coA hydrolysis assay | Bioorg Med Chem Lett 21: 1185-90 (2011) Article DOI: 10.1016/j.bmcl.2010.12.099 BindingDB Entry DOI: 10.7270/Q2SJ1KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylamine N-acetyltransferase (Pseudomonas aeruginosa) | BDBM50336283 (3-[3'-(4-cyclopent-2'''-en-1'''-ylphenoxy)-2'-hydr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa NAT using 5-aminosalicylate substrate by acetyl-coA hydrolysis assay | Bioorg Med Chem Lett 21: 1185-90 (2011) Article DOI: 10.1016/j.bmcl.2010.12.099 BindingDB Entry DOI: 10.7270/Q2SJ1KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylamine N-acetyltransferase (Mycobacterium smegmatis) | BDBM50336283 (3-[3'-(4-cyclopent-2'''-en-1'''-ylphenoxy)-2'-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis NAT using isoniazed substrate by acetyl-coA hydrolysis assay | Bioorg Med Chem Lett 21: 1185-90 (2011) Article DOI: 10.1016/j.bmcl.2010.12.099 BindingDB Entry DOI: 10.7270/Q2SJ1KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylamine N-acetyltransferase (Mycobacterium smegmatis) | BDBM50336283 (3-[3'-(4-cyclopent-2'''-en-1'''-ylphenoxy)-2'-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis NAT using 5-aminosalicylate substrate by acetyl-coA hydrolysis assay | Bioorg Med Chem Lett 21: 1185-90 (2011) Article DOI: 10.1016/j.bmcl.2010.12.099 BindingDB Entry DOI: 10.7270/Q2SJ1KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trehalose-binding lipoprotein LpqY (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50235450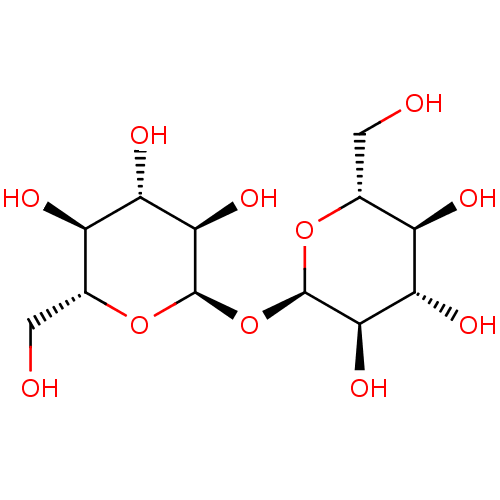 (CHEBI:16551 | TREHALOSE) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00104g BindingDB Entry DOI: 10.7270/Q2TQ65JT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trehalose-binding lipoprotein LpqY (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50574428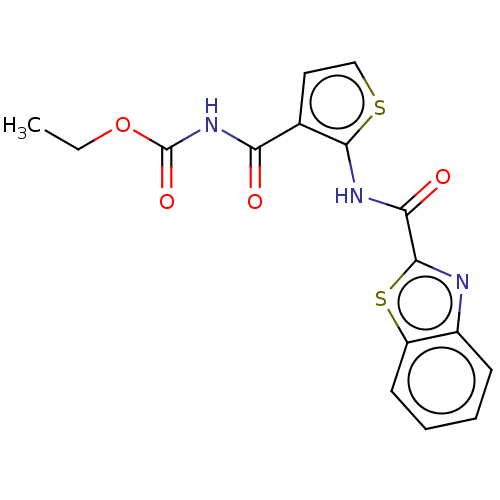 (CHEMBL3262462) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00104g BindingDB Entry DOI: 10.7270/Q2TQ65JT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trehalose-binding lipoprotein LpqY (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50075050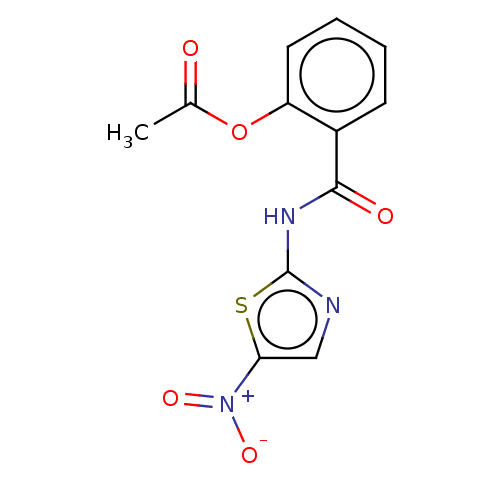 (Alinia | Nitazoxanide | PH-5776) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.54E+4 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00104g BindingDB Entry DOI: 10.7270/Q2TQ65JT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||