

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239385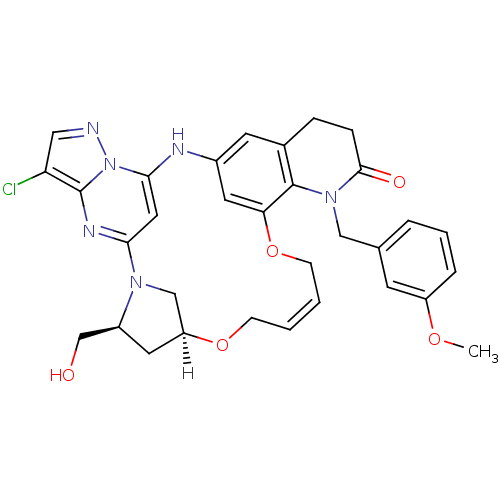 (CHEMBL4092565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor in guinea pig cerebral cortical membranes by displacement of [3H]- WB-4101 | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239368 (CHEMBL4073463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239368 (CHEMBL4073463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239362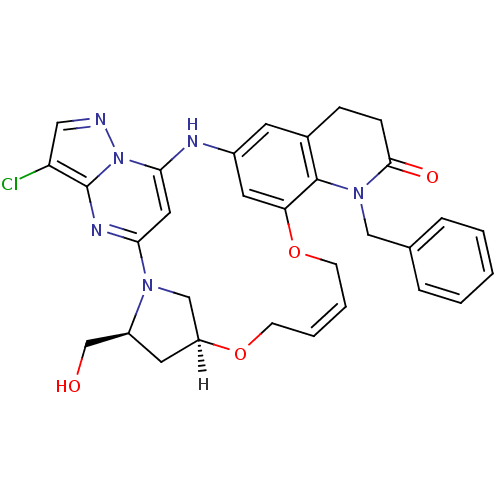 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239362 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533556 (US11225475, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533555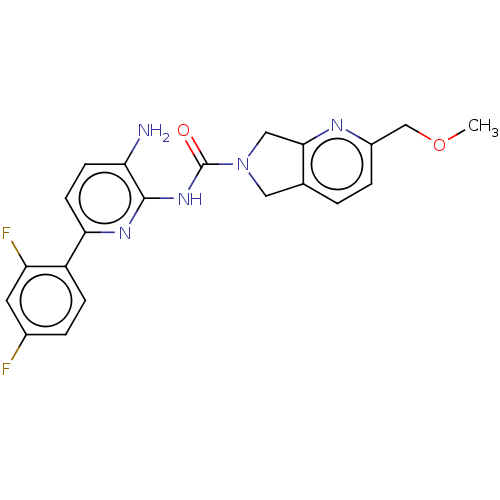 (US11225475, Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533539 (US11225475, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM533539 (US11225475, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465367 (US10793567, Compound 1 | US11225479, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465368 (US10793567, Compound 2 | US11225479, Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465369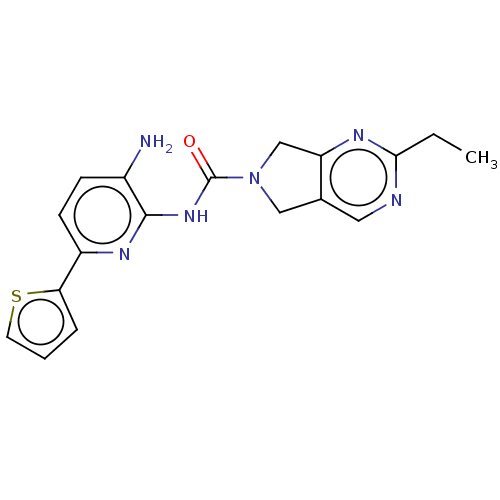 (US10793567, Compound 3 | US11225479, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465371 (US10793567, Compound 5 | US11225479, Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM533734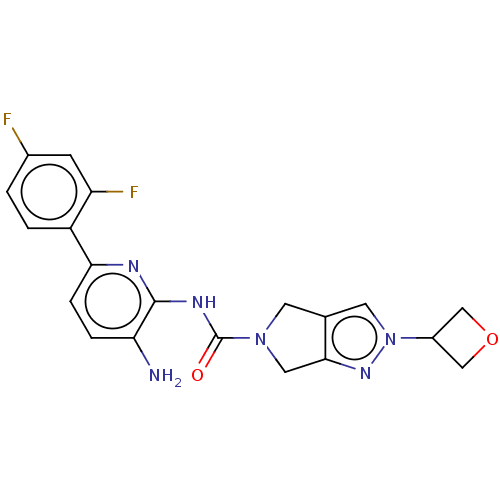 (US11225479, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533734 (US11225479, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM465374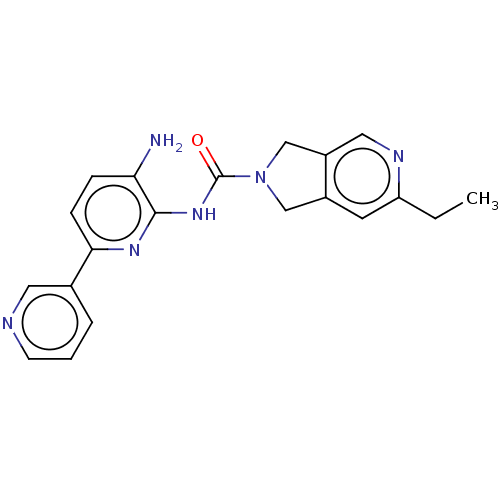 (US10793567, Compound 8 | US11225479, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465374 (US10793567, Compound 8 | US11225479, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465375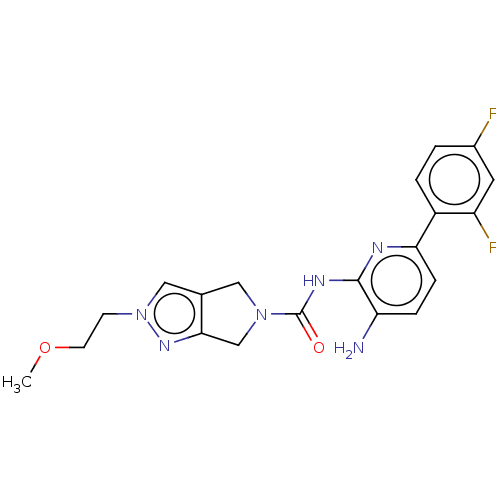 (US10793567, Compound 9 | US11225479, Compound 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465378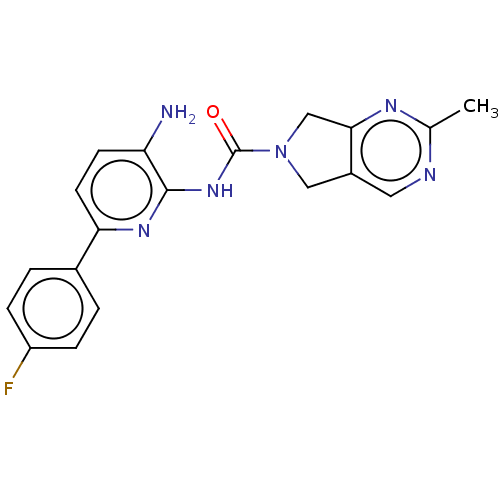 (US10793567, Compound 14 | US11225479, Compound 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM465381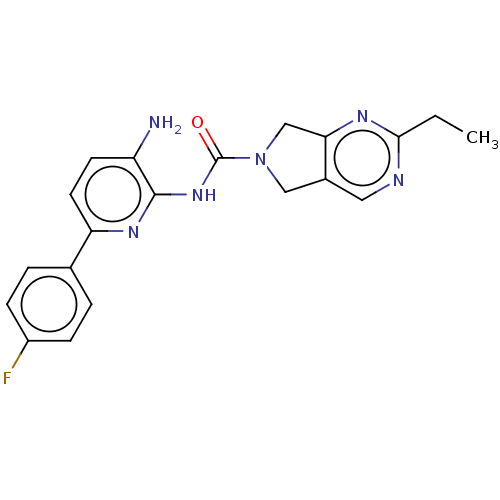 (US10793567, Compound 17 | US11225479, Compound 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465381 (US10793567, Compound 17 | US11225479, Compound 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H998DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533559 (US11225475, Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533571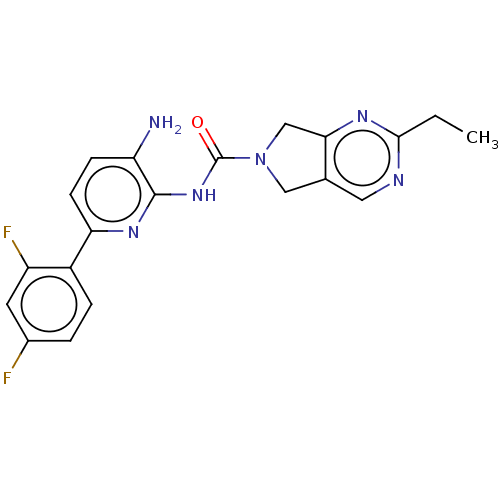 (US11225475, Compound 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533574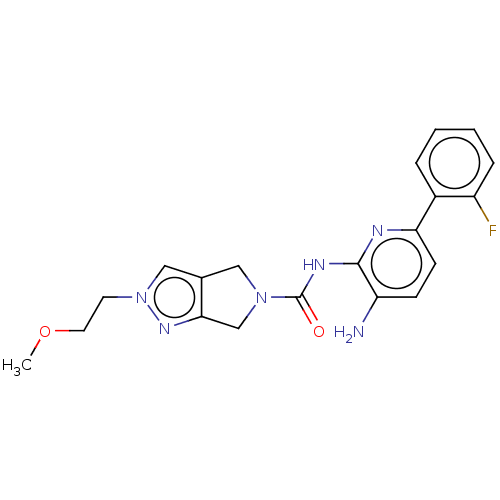 (US11225475, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533582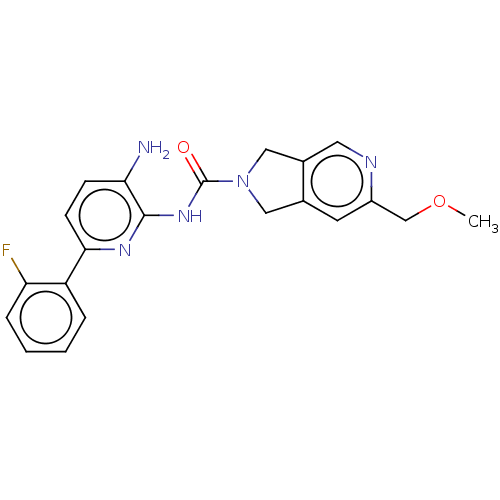 (US11225475, Compound 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM533585 (US11225475, Compound 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM533585 (US11225475, Compound 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30156 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239380 (CHEMBL4062105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239380 (CHEMBL4062105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239383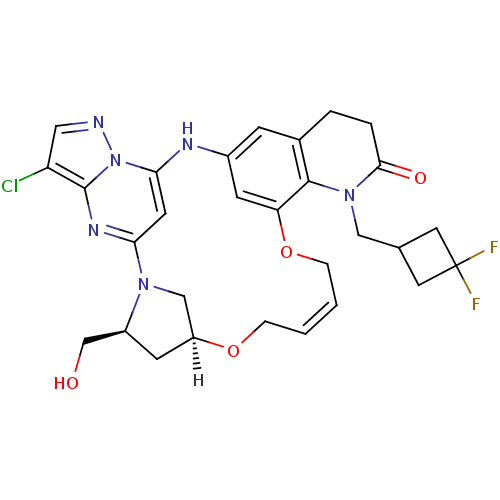 (CHEMBL4096773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239383 (CHEMBL4096773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239379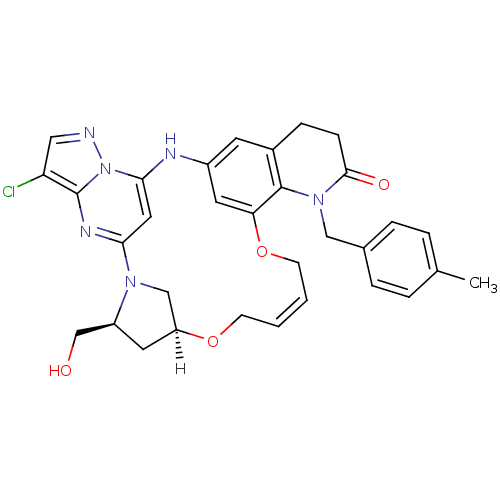 (CHEMBL4083842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239379 (CHEMBL4083842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50065241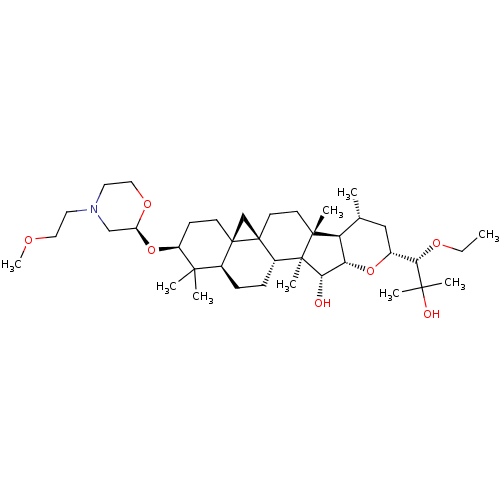 (CHEMBL2207044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Satori Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 5 mins before substrate addition by LC-MS/MS analysis | Bioorg Med Chem Lett 25: 1621-6 (2015) Article DOI: 10.1016/j.bmcl.2015.01.051 BindingDB Entry DOI: 10.7270/Q2JW8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239367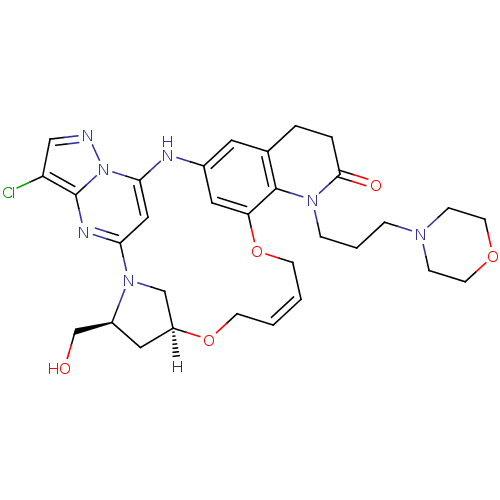 (CHEMBL4084806) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465375 (US10793567, Compound 9 | US11225479, Compound 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465374 (US10793567, Compound 8 | US11225479, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM465374 (US10793567, Compound 8 | US11225479, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465372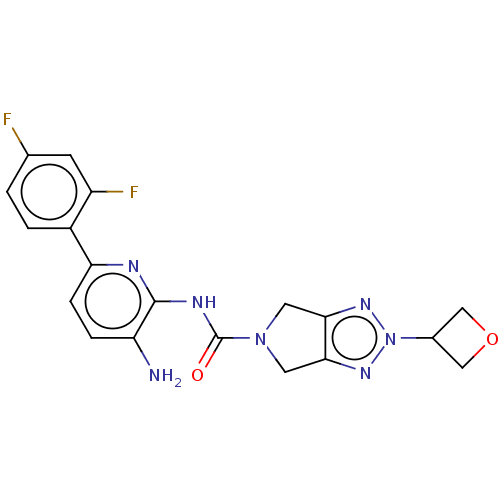 (US10793567, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM465372 (US10793567, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465371 (US10793567, Compound 5 | US11225479, Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465369 (US10793567, Compound 3 | US11225479, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465368 (US10793567, Compound 2 | US11225479, Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465367 (US10793567, Compound 1 | US11225479, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239367 (CHEMBL4084806) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465378 (US10793567, Compound 14 | US11225479, Compound 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM465381 (US10793567, Compound 17 | US11225479, Compound 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM465381 (US10793567, Compound 17 | US11225479, Compound 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description The following describes an assay protocol for measuring the deacetylation of a peptide substrate by the enzymes HDAC2 or HDAC1. Enzyme, substrate, an... | US Patent US10793567 (2020) BindingDB Entry DOI: 10.7270/Q2542RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 205 total ) | Next | Last >> |