

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50384416 (CHEMBL2111553 | CHEMBL291536 | SB-334867) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Binding affinity to adenosine 2A receptor (unknown origin) | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50384416 (CHEMBL2111553 | CHEMBL291536 | SB-334867) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Binding affinity to 5HT2c receptor (unknown origin) | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208487 (US9266870, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260997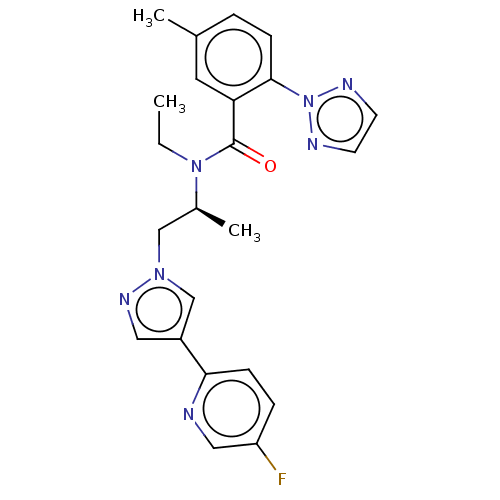 (CHEMBL4073607) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.767 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543836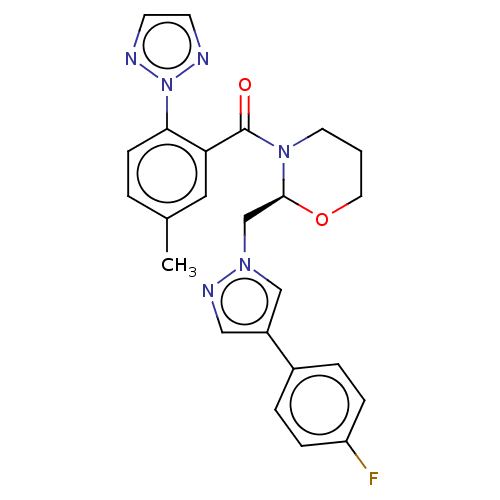 (CHEMBL4637711) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.784 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208524 (US9266870, 40 | US9266870, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208519 (US9266870, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50261002 (CHEMBL4059968) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208511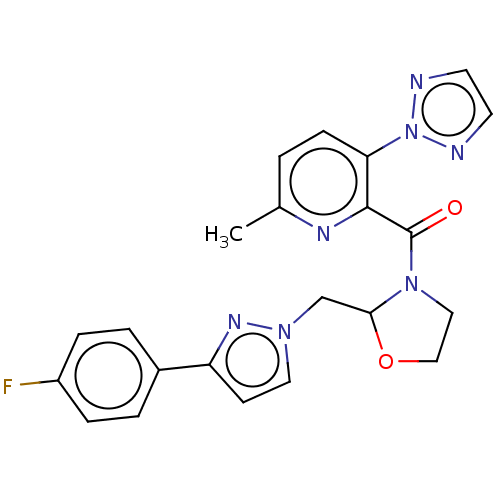 (US9266870, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208525 (US9266870, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208519 (US9266870, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208525 (US9266870, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50543827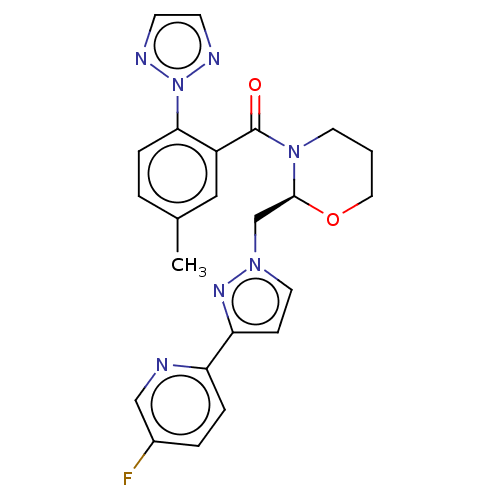 (CHEMBL4638046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260978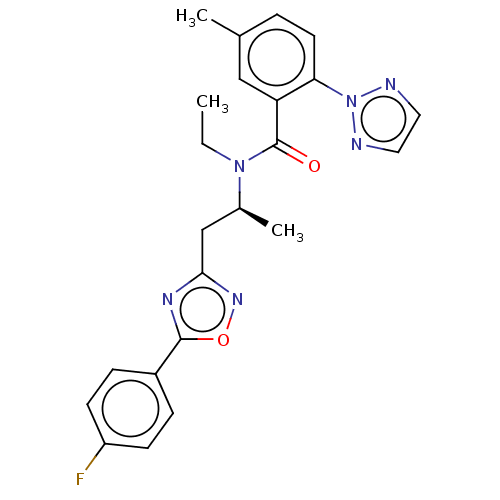 (CHEMBL4091475) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260979 (CHEMBL4069535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208521 (US9266870, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208513 (US9266870, 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208487 (US9266870, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260983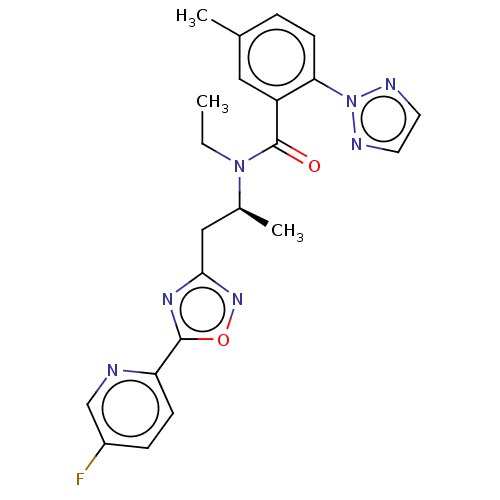 (CHEMBL4089060) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260976 (CHEMBL4081186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543827 (CHEMBL4638046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260963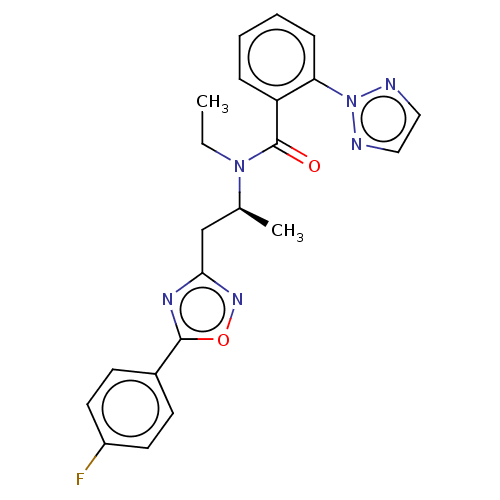 (CHEMBL4077821) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208524 (US9266870, 40 | US9266870, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260977 (CHEMBL4070565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543819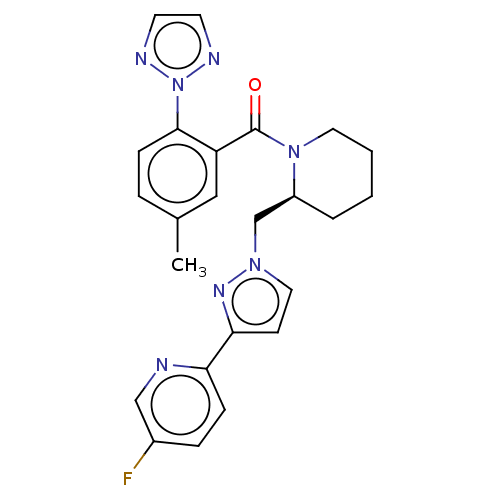 (CHEMBL4639148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260985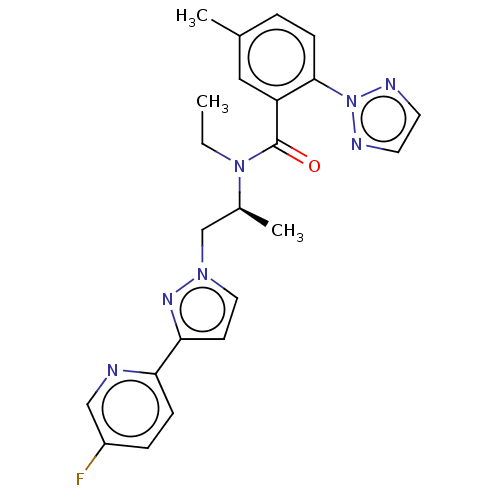 (CHEMBL4100428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260975 (CHEMBL4095852) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50260985 (CHEMBL4100428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX2R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208521 (US9266870, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50261019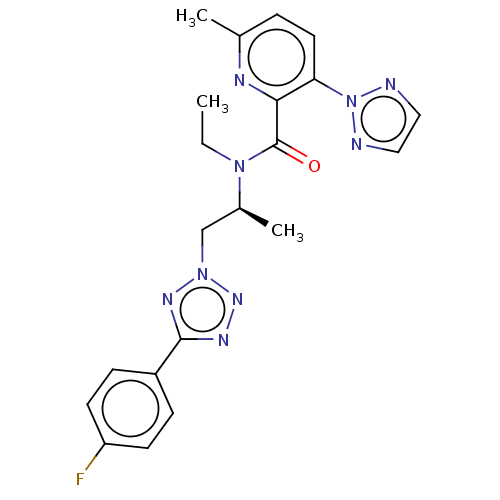 (CHEMBL4087320) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50261003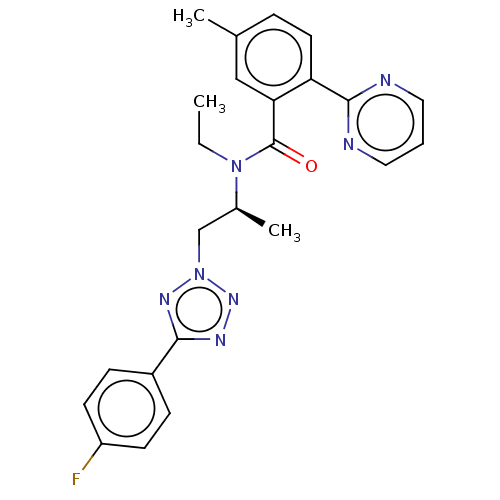 (CHEMBL4060971) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50261000 (CHEMBL4080357) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543833 (CHEMBL4634034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260984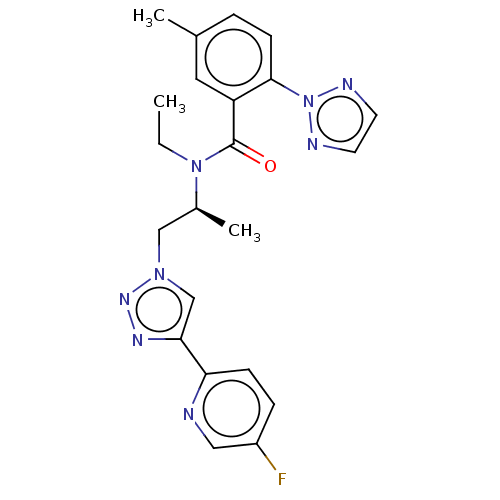 (CHEMBL4060868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50260987 (CHEMBL4088347) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543837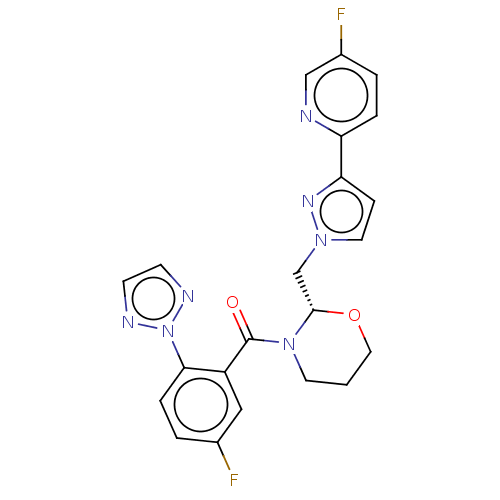 (CHEMBL4641191) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543831 (CHEMBL4637497) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50261005 (CHEMBL4090659) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208522 (US9266870, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50543836 (CHEMBL4637711) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543830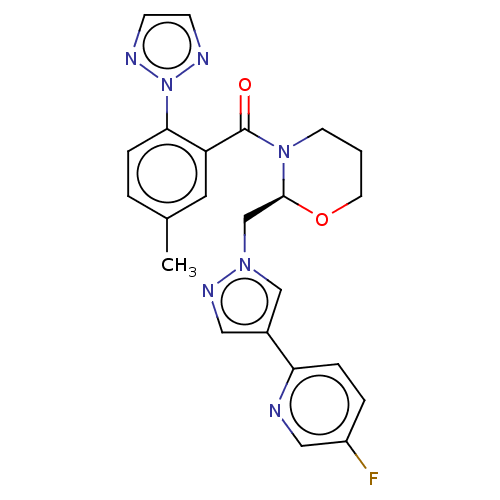 (CHEMBL4642480) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208518 (US9266870, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50543819 (CHEMBL4639148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208512 (US9266870, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208501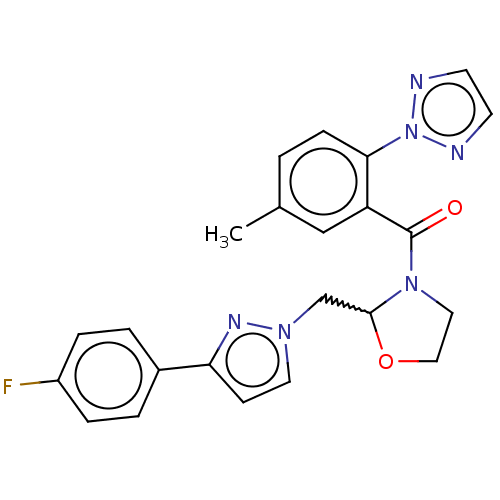 (US9266870, 17 | US9266870, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208501 (US9266870, 17 | US9266870, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50261006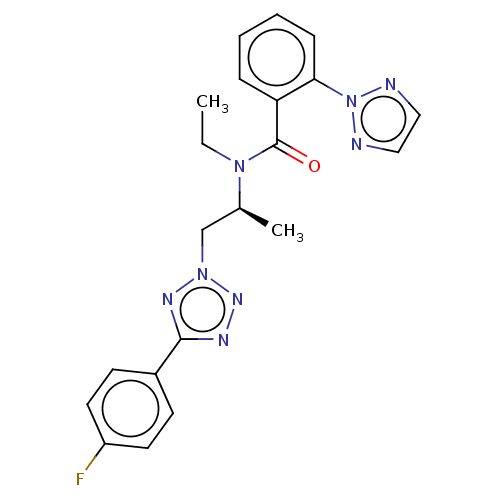 (CHEMBL4065909) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemistry Laboratories, Taisho Pharmaceutical Co., Ltd., 1-403 Yoshino-cho, Kita-ku, Saitama 331-9530, Japan. Electronic address: a-futamura@so.taisho.co.jp. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as reduction in [Ala6,12]orexin-A-induced intracellular Ca2+ mobilization incubated... | Bioorg Med Chem 25: 5203-5215 (2017) Article DOI: 10.1016/j.bmc.2017.07.051 BindingDB Entry DOI: 10.7270/Q2D50QD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543838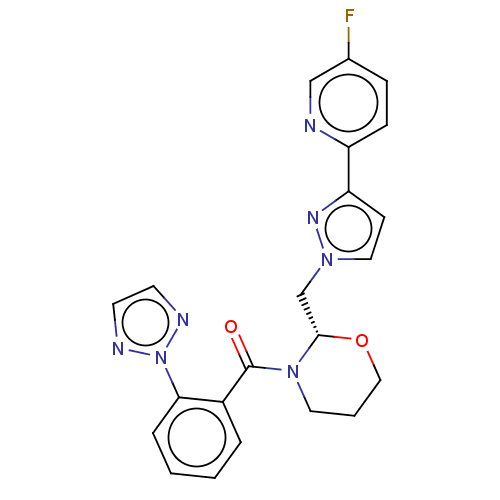 (CHEMBL4633158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208514 (US9266870, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208527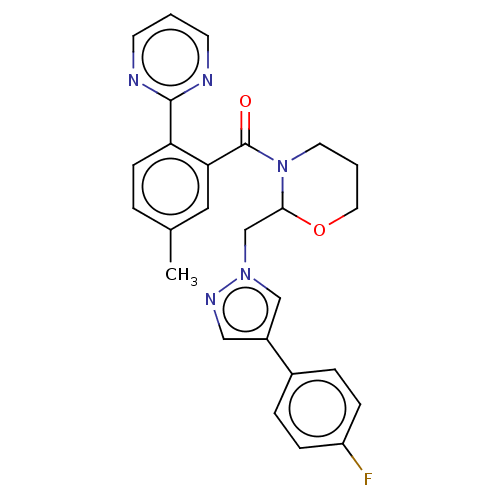 (US9266870, 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 222 total ) | Next | Last >> |