 Found 2273 hits with Last Name = 'gall' and Initial = 'a'
Found 2273 hits with Last Name = 'gall' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50018830
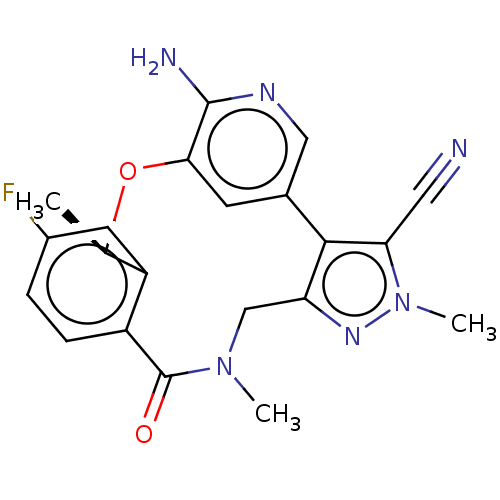
(CHEMBL3286830 | US10543199, Compound PF-06463922 |...)Show SMILES C[C@H]1Oc2cc(cnc2N)-c2c(CN(C)C(=O)c3ccc(F)cc13)nn(C)c2C#N |r| Show InChI InChI=1S/C21H19FN6O2/c1-11-15-7-13(22)4-5-14(15)21(29)27(2)10-16-19(17(8-23)28(3)26-16)12-6-18(30-11)20(24)25-9-12/h4-7,9,11H,10H2,1-3H3,(H2,24,25)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ROS1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00147
BindingDB Entry DOI: 10.7270/Q2D2227R |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071565
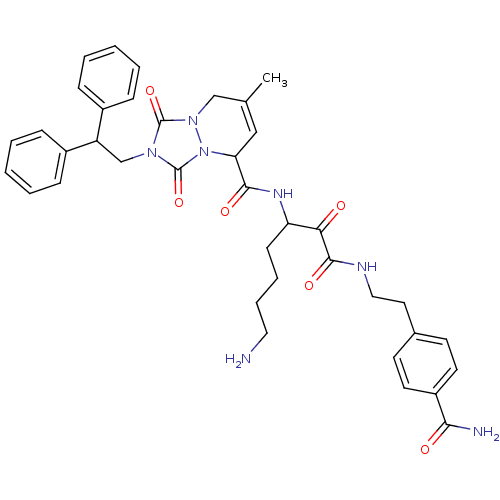
(2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...)Show SMILES CC1=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc2ccc(cc2)C(N)=O)n2n(C1)c(=O)n(CC(c1ccccc1)c1ccccc1)c2=O |t:1| Show InChI InChI=1S/C38H43N7O6/c1-25-22-32(35(48)42-31(14-8-9-20-39)33(46)36(49)41-21-19-26-15-17-29(18-16-26)34(40)47)45-38(51)43(37(50)44(45)23-25)24-30(27-10-4-2-5-11-27)28-12-6-3-7-13-28/h2-7,10-13,15-18,22,30-32H,8-9,14,19-21,23-24,39H2,1H3,(H2,40,47)(H,41,49)(H,42,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519706
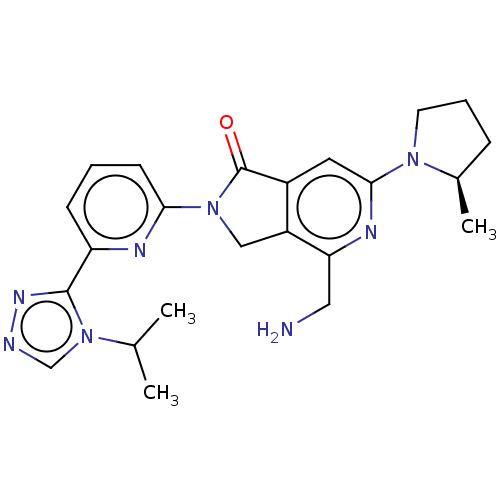
(US11142525, Example 107)Show SMILES CC(C)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606126
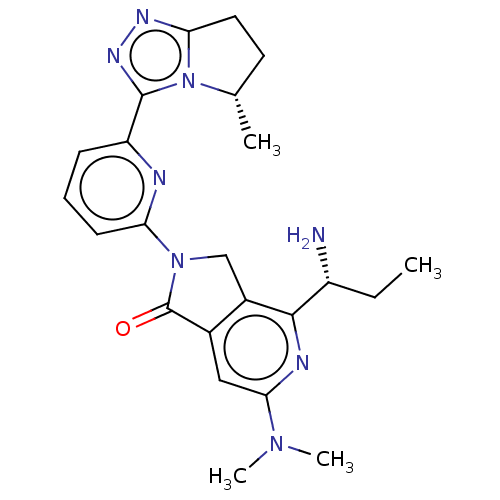
(4-[(1R)-1- aminopropyl]- 6-(dimethyl- amino)-2-{6-...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519743

(US11142525, Example 144)Show SMILES CCC(CC)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519602

(US11142525, Example 18)Show SMILES CC[C@@H](CC(F)(F)F)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N(C)C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606137
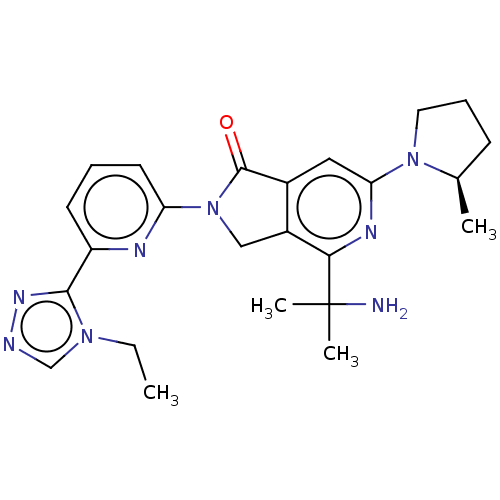
(US11684616, Example 100)Show SMILES CCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2C(C)(C)N)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606194

(US11684616, Example 200)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4CNC)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606195

(US11684616, Example 201)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4CNC)N(C)C(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606199

(2-{6-[(5R)-5- (fluoromethyl)- 6,7-dihydro-5H- pyrr...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606204

(4-[(methyl- amino)methyl]- 2-{6-[(5S)- 5-methyl-6,...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606205

(2-{6-[(5S,7S)- 5,7-dimethyl-6,7- dihydro-5H- pyrro...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2[C@@H](C)C[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606128
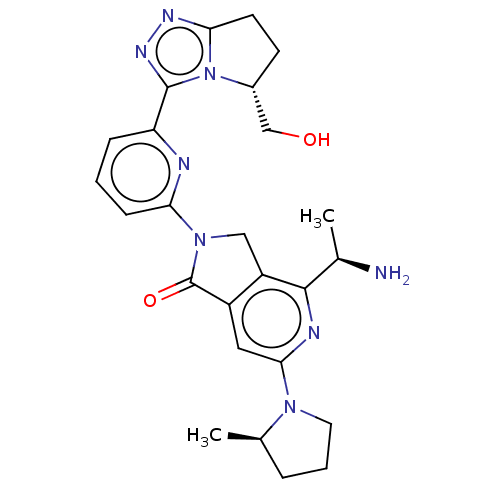
(4-[(1R)-1- aminoethyl]-2- {6-[(5R)-5- (hydroxy- me...)Show SMILES C[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606129

(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (hydroxy- m...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519590

(US11142525, Example 6 | US11142525, Example 79)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1C(C)C)N(C)C(C)C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519699

(US11142525, Example 100)Show SMILES CC(C)N(C)c1cc2C(=O)N(Cc2c(CN)n1)c1cccc(n1)N1[C@@H](C)COC1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606115

(4-(2- aminopropan- 2-yl)-2-{6- [(5S)-5-methyl- 6,7...)Show SMILES C[C@@H]1CCCN1c1cc2C(=O)N(Cc2c(n1)C(C)(C)N)c1cccc(n1)-c1nnc2CC[C@H](C)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606114

(4-[(1S)-1- aminopropyl]- 2-{6-[(5$#958;)-5- ethyl-...)Show SMILES CC[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](CC)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606118

(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (hydroxy- m...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606123

(4-[(1$#958;)-1- aminoethyl]-2- {6-[(5$#958;)-5- me...)Show SMILES C[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606098
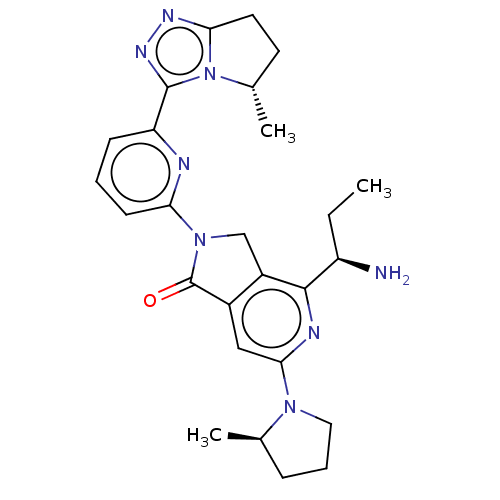
(US11684616, Example 1)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606099

(US11684616, Example 2)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@@H](C)N)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606100
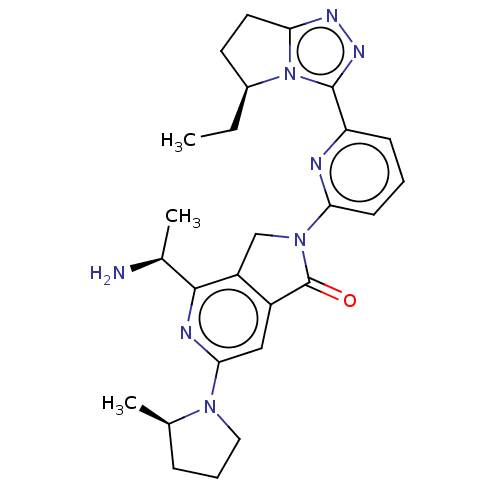
(US11684616, Example 3)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@H](C)N)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606101

(US11684616, Example 4)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@@H](C)N)N(C)C(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606102

(US11684616, Example 5)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606125

(4-[(1$#958;)-1- aminoethyl]-2- {6-[(5$#958;)-5- me...)Show SMILES C[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519601

(US11142525, Example 17)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1[C@@H](C)CC(F)(F)F)N(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606135
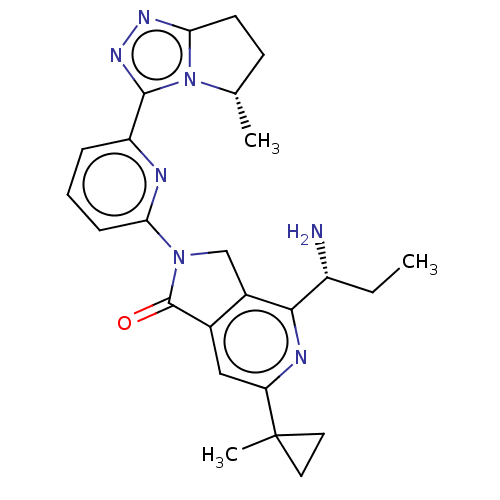
(4-[(1$#958;)-1- aminopropyl]- 6-(1- methylcyclo- p...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)C1(C)CC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519746

(US11142525, Example 150)Show SMILES CCC(CC)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N(C)C(C)C)C1=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519770
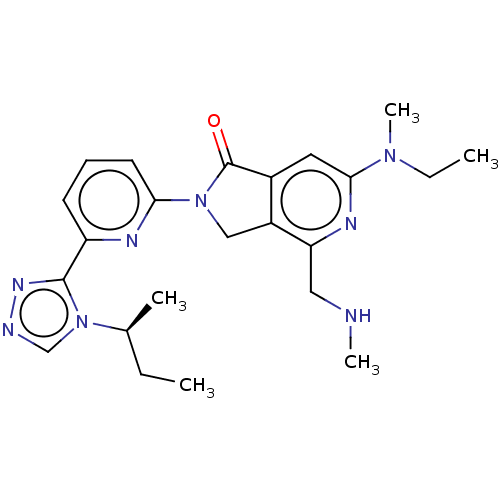
(US11142525, Example 173)Show SMILES CC[C@H](C)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N(C)CC)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606196

(US11684616, Example 202)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519708

(US11142525, Example 109)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1C(C)C)N1CCCC1(C)C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519653
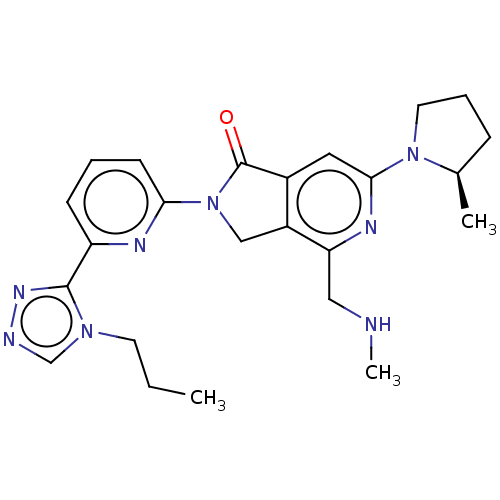
(US11142525, Example 55)Show SMILES CCCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606130
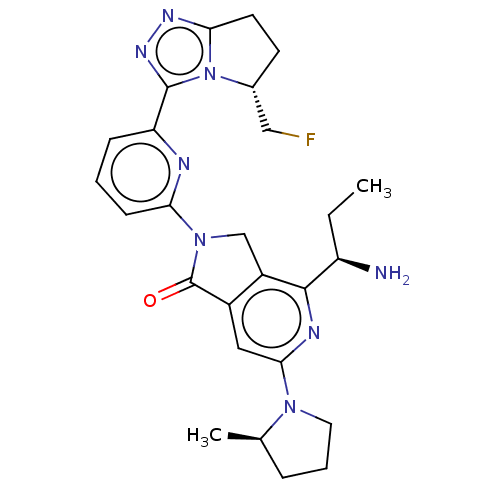
(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (fluorometh...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519673

(US11142525, Example 74)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)N1[C@@H](C)COC1=O)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606119
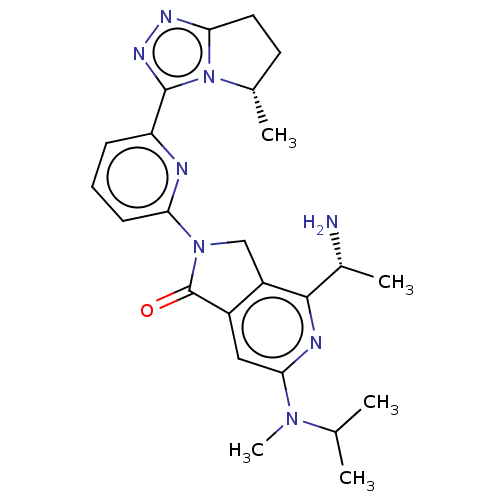
(4-[(1R)-1- aminoethyl]-2- {6-[(5S)-5- methyl-6,7- ...)Show SMILES CC(C)N(C)c1cc2C(=O)N(Cc2c(n1)[C@@H](C)N)c1cccc(n1)-c1nnc2CC[C@H](C)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606178

(4-[(1R)-1- aminopropyl]- 2-[6-(4-ethyl- 4H-1,2,4- ...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1CC)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519667

(US11142525, Example 68)Show SMILES C[C@@H]1CCCN1c1cc2C(=O)N(Cc2c(CN)n1)c1cccc(n1)N1[C@@H](CF)COC1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519599
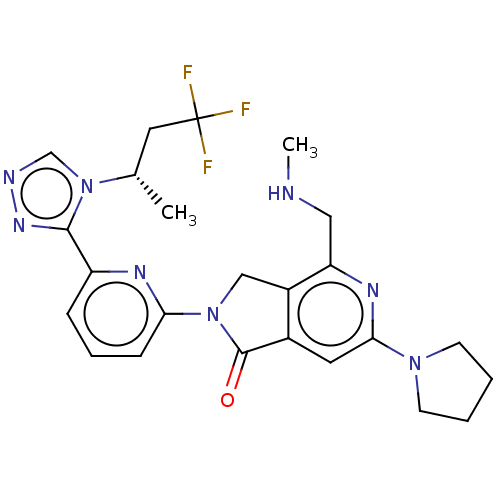
(US11142525, Example 15)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1[C@@H](C)CC(F)(F)F)N1CCCC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606111

(4-[(1R)-1- aminopropyl]- 2-{6-[(5$#958;)-5- ethyl-...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](CC)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606134

(4-[(1$#958;)-1- aminopropyl]- 2-{6-[(5R)-5- (hydro...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)C1(C)CC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519688
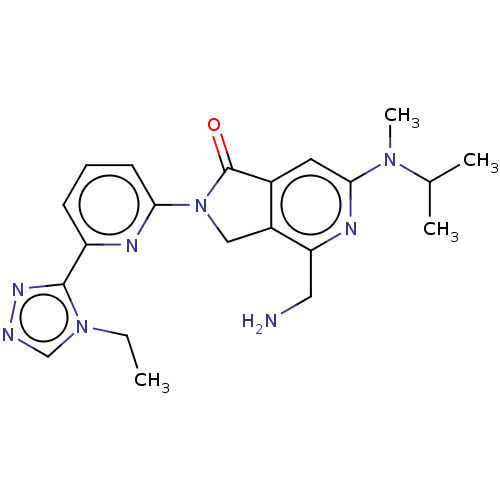
(US11142525, Example 89)Show SMILES CCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N(C)C(C)C)C1=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50166996

(2-(4-Methyl-piperazin-1-yl)-3-propyl-quinoline-4-c...)Show InChI InChI=1S/C20H27N3O2/c1-4-8-16-18(20(24)25-5-2)15-9-6-7-10-17(15)21-19(16)23-13-11-22(3)12-14-23/h6-7,9-10H,4-5,8,11-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane |
J Med Chem 48: 3564-75 (2005)
Article DOI: 10.1021/jm0493461
BindingDB Entry DOI: 10.7270/Q2HH6KTH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519597
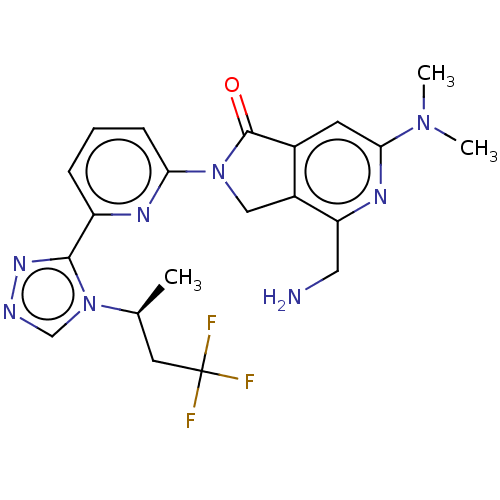
(US11142525, Example 13)Show SMILES C[C@@H](CC(F)(F)F)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N(C)C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606120
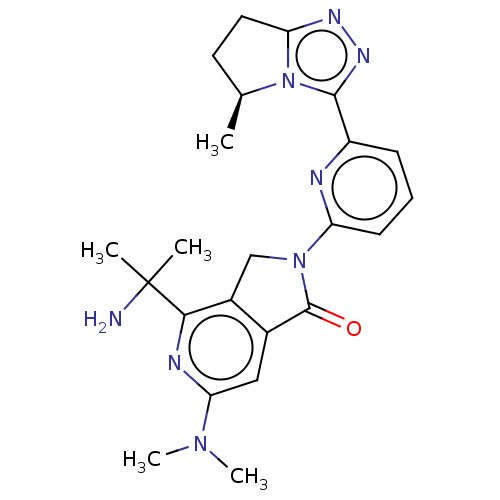
(4-(2- aminopropan- 2-yl)-6- (dimethyl- amino)-2-{6...)Show SMILES C[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4C(C)(C)N)N(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519719

(US11142525, Example 120)Show SMILES CCCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@@H]2C(F)(F)F)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519720

(US11142525, Example 121)Show SMILES CCn1c(C)nnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519737
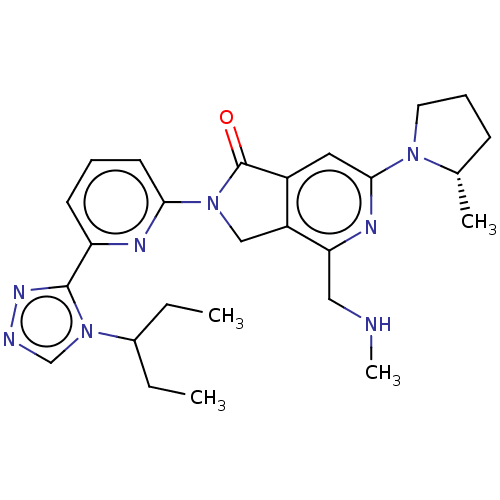
(US11142525, Example 138)Show SMILES CCC(CC)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519742
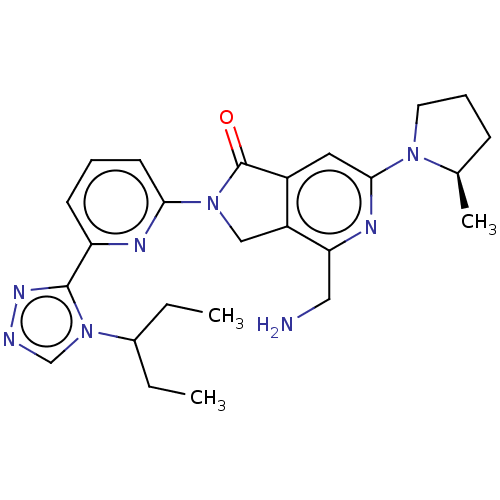
(US11142525, Example 143)Show SMILES CCC(CC)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519714

(US11142525, Example 115)Show SMILES CCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data