 Found 101 hits with Last Name = 'gomez' and Initial = 'rc'
Found 101 hits with Last Name = 'gomez' and Initial = 'rc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50273389
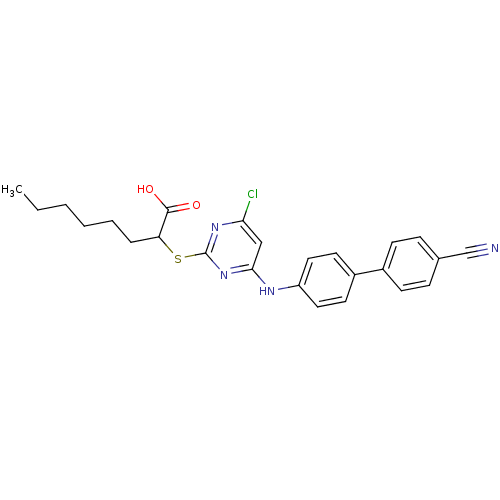
(2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PPARalpha ligand binding domain expressed in COS7 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443842
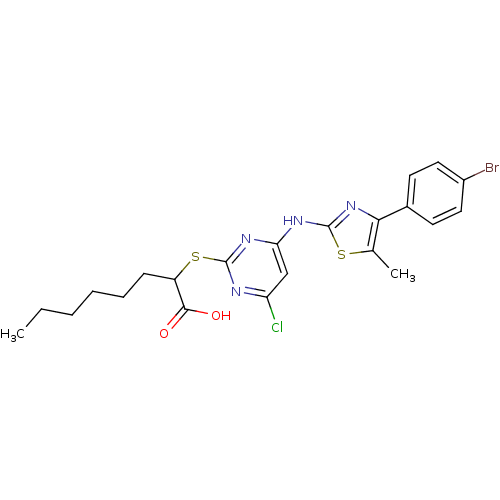
(CHEMBL3094426)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(c(C)s2)-c2ccc(Br)cc2)n1)C(O)=O Show InChI InChI=1S/C22H24BrClN4O2S2/c1-3-4-5-6-7-16(20(29)30)32-21-25-17(24)12-18(26-21)27-22-28-19(13(2)31-22)14-8-10-15(23)11-9-14/h8-12,16H,3-7H2,1-2H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443839
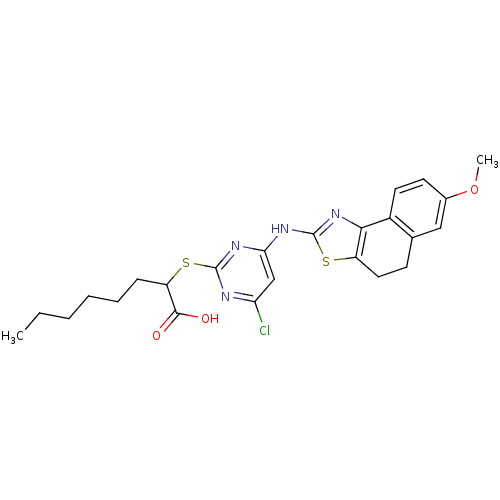
(CHEMBL3094410)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc-3c(CCc4cc(OC)ccc-34)s2)n1)C(O)=O Show InChI InChI=1S/C24H27ClN4O3S2/c1-3-4-5-6-7-18(22(30)31)34-23-26-19(25)13-20(27-23)28-24-29-21-16-10-9-15(32-2)12-14(16)8-11-17(21)33-24/h9-10,12-13,18H,3-8,11H2,1-2H3,(H,30,31)(H,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443828
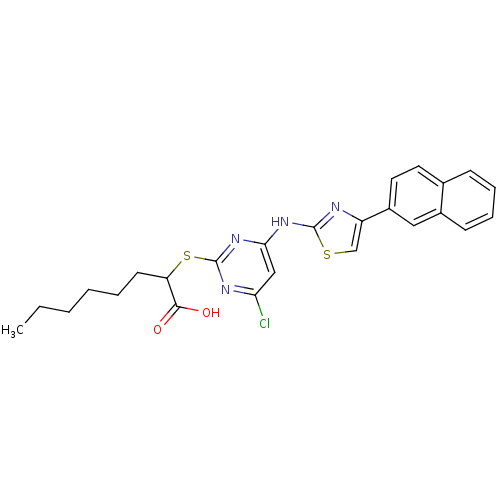
(CHEMBL3094412)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc3ccccc3c2)n1)C(O)=O Show InChI InChI=1S/C25H25ClN4O2S2/c1-2-3-4-5-10-20(23(31)32)34-25-28-21(26)14-22(30-25)29-24-27-19(15-33-24)18-12-11-16-8-6-7-9-17(16)13-18/h6-9,11-15,20H,2-5,10H2,1H3,(H,31,32)(H,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443831
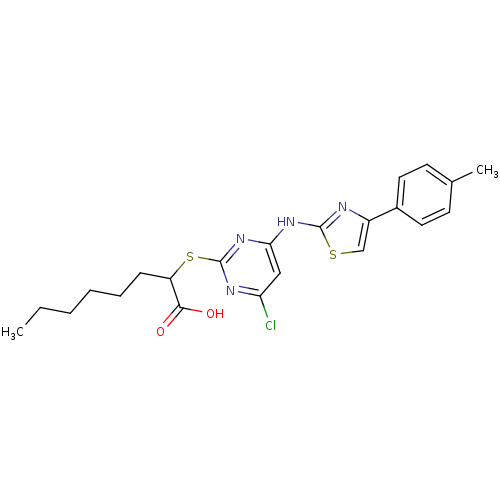
(CHEMBL3091499)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(C)cc2)n1)C(O)=O Show InChI InChI=1S/C22H25ClN4O2S2/c1-3-4-5-6-7-17(20(28)29)31-22-25-18(23)12-19(27-22)26-21-24-16(13-30-21)15-10-8-14(2)9-11-15/h8-13,17H,3-7H2,1-2H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443828

(CHEMBL3094412)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc3ccccc3c2)n1)C(O)=O Show InChI InChI=1S/C25H25ClN4O2S2/c1-2-3-4-5-10-20(23(31)32)34-25-28-21(26)14-22(30-25)29-24-27-19(15-33-24)18-12-11-16-8-6-7-9-17(16)13-18/h6-9,11-15,20H,2-5,10H2,1H3,(H,31,32)(H,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443822
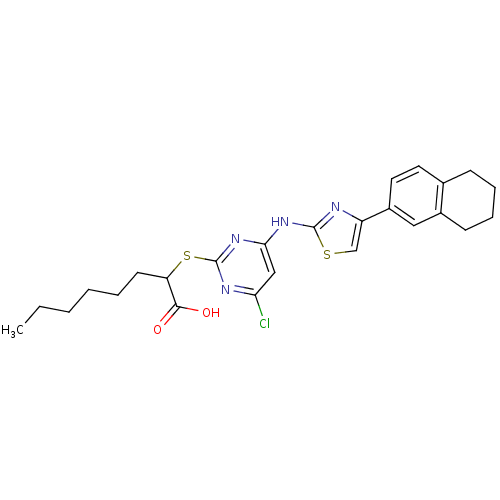
(CHEMBL3094418)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc3CCCCc3c2)n1)C(O)=O Show InChI InChI=1S/C25H29ClN4O2S2/c1-2-3-4-5-10-20(23(31)32)34-25-28-21(26)14-22(30-25)29-24-27-19(15-33-24)18-12-11-16-8-6-7-9-17(16)13-18/h11-15,20H,2-10H2,1H3,(H,31,32)(H,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443828

(CHEMBL3094412)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc3ccccc3c2)n1)C(O)=O Show InChI InChI=1S/C25H25ClN4O2S2/c1-2-3-4-5-10-20(23(31)32)34-25-28-21(26)14-22(30-25)29-24-27-19(15-33-24)18-12-11-16-8-6-7-9-17(16)13-18/h6-9,11-15,20H,2-5,10H2,1H3,(H,31,32)(H,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443822

(CHEMBL3094418)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc3CCCCc3c2)n1)C(O)=O Show InChI InChI=1S/C25H29ClN4O2S2/c1-2-3-4-5-10-20(23(31)32)34-25-28-21(26)14-22(30-25)29-24-27-19(15-33-24)18-12-11-16-8-6-7-9-17(16)13-18/h11-15,20H,2-10H2,1H3,(H,31,32)(H,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443815

(CHEMBL3094425)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(c(C)s2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C22H25ClN4O2S2/c1-3-4-5-9-12-16(20(28)29)31-21-24-17(23)13-18(25-21)26-22-27-19(14(2)30-22)15-10-7-6-8-11-15/h6-8,10-11,13,16H,3-5,9,12H2,1-2H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443832

(CHEMBL3091498)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C21H23ClN4O2S2/c1-2-3-4-8-11-16(19(27)28)30-21-24-17(22)12-18(26-21)25-20-23-15(13-29-20)14-9-6-5-7-10-14/h5-7,9-10,12-13,16H,2-4,8,11H2,1H3,(H,27,28)(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443831

(CHEMBL3091499)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(C)cc2)n1)C(O)=O Show InChI InChI=1S/C22H25ClN4O2S2/c1-3-4-5-6-7-17(20(28)29)31-22-25-18(23)12-19(27-22)26-21-24-16(13-30-21)15-10-8-14(2)9-11-15/h8-13,17H,3-7H2,1-2H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443815

(CHEMBL3094425)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(c(C)s2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C22H25ClN4O2S2/c1-3-4-5-9-12-16(20(28)29)31-21-24-17(23)13-18(25-21)26-22-27-19(14(2)30-22)15-10-7-6-8-11-15/h6-8,10-11,13,16H,3-5,9,12H2,1-2H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443838

(CHEMBL3094431)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C21H22Cl2N4O2S2/c1-2-3-4-5-6-16(19(28)29)31-21-25-17(23)11-18(27-21)26-20-24-15(12-30-20)13-7-9-14(22)10-8-13/h7-12,16H,2-6H2,1H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443838

(CHEMBL3094431)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C21H22Cl2N4O2S2/c1-2-3-4-5-6-16(19(28)29)31-21-25-17(23)11-18(27-21)26-20-24-15(12-30-20)13-7-9-14(22)10-8-13/h7-12,16H,2-6H2,1H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443832

(CHEMBL3091498)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C21H23ClN4O2S2/c1-2-3-4-8-11-16(19(27)28)30-21-24-17(22)12-18(26-21)25-20-23-15(13-29-20)14-9-6-5-7-10-14/h5-7,9-10,12-13,16H,2-4,8,11H2,1H3,(H,27,28)(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443837
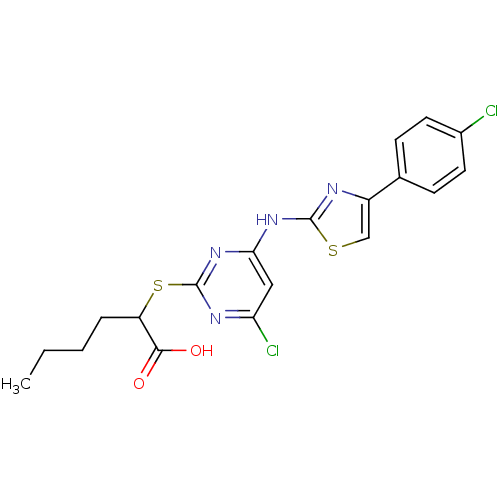
(CHEMBL3094432)Show SMILES CCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C19H18Cl2N4O2S2/c1-2-3-4-14(17(26)27)29-19-23-15(21)9-16(25-19)24-18-22-13(10-28-18)11-5-7-12(20)8-6-11/h5-10,14H,2-4H2,1H3,(H,26,27)(H,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443838

(CHEMBL3094431)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C21H22Cl2N4O2S2/c1-2-3-4-5-6-16(19(28)29)31-21-25-17(23)11-18(27-21)26-20-24-15(12-30-20)13-7-9-14(22)10-8-13/h7-12,16H,2-6H2,1H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM24560
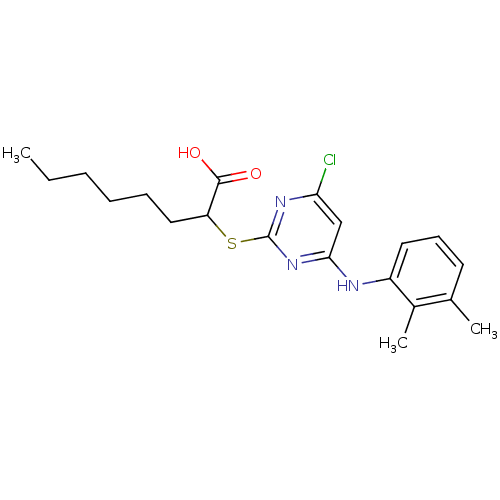
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cccc(C)c2C)n1)C(O)=O Show InChI InChI=1S/C20H26ClN3O2S/c1-4-5-6-7-11-16(19(25)26)27-20-23-17(21)12-18(24-20)22-15-10-8-9-13(2)14(15)3/h8-10,12,16H,4-7,11H2,1-3H3,(H,25,26)(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PPARalpha ligand binding domain expressed in COS7 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443837

(CHEMBL3094432)Show SMILES CCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C19H18Cl2N4O2S2/c1-2-3-4-14(17(26)27)29-19-23-15(21)9-16(25-19)24-18-22-13(10-28-18)11-5-7-12(20)8-6-11/h5-10,14H,2-4H2,1H3,(H,26,27)(H,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443842

(CHEMBL3094426)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(c(C)s2)-c2ccc(Br)cc2)n1)C(O)=O Show InChI InChI=1S/C22H24BrClN4O2S2/c1-3-4-5-6-7-16(20(29)30)32-21-25-17(24)12-18(26-21)27-22-28-19(13(2)31-22)14-8-10-15(23)11-9-14/h8-12,16H,3-7H2,1-2H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443836

(CHEMBL3094433)Show SMILES CCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C17H14Cl2N4O2S2/c1-2-12(15(24)25)27-17-21-13(19)7-14(23-17)22-16-20-11(8-26-16)9-3-5-10(18)6-4-9/h3-8,12H,2H2,1H3,(H,24,25)(H,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443823

(CHEMBL3094417)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(cc2)[N+]([O-])=O)n1)C(O)=O Show InChI InChI=1S/C21H22ClN5O4S2/c1-2-3-4-5-6-16(19(28)29)33-21-24-17(22)11-18(26-21)25-20-23-15(12-32-20)13-7-9-14(10-8-13)27(30)31/h7-12,16H,2-6H2,1H3,(H,28,29)(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443825

(CHEMBL3094415)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(F)c(F)c2)n1)C(O)=O Show InChI InChI=1S/C21H21ClF2N4O2S2/c1-2-3-4-5-6-16(19(29)30)32-21-26-17(22)10-18(28-21)27-20-25-15(11-31-20)12-7-8-13(23)14(24)9-12/h7-11,16H,2-6H2,1H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443824

(CHEMBL3094416)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(F)cc2F)n1)C(O)=O Show InChI InChI=1S/C21H21ClF2N4O2S2/c1-2-3-4-5-6-16(19(29)30)32-21-26-17(22)10-18(28-21)27-20-25-15(11-31-20)13-8-7-12(23)9-14(13)24/h7-11,16H,2-6H2,1H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50273389

(2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PPARgamma ligand binding domain expressed in COS7 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443842

(CHEMBL3094426)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(c(C)s2)-c2ccc(Br)cc2)n1)C(O)=O Show InChI InChI=1S/C22H24BrClN4O2S2/c1-3-4-5-6-7-16(20(29)30)32-21-25-17(24)12-18(26-21)27-22-28-19(13(2)31-22)14-8-10-15(23)11-9-14/h8-12,16H,3-7H2,1-2H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443825

(CHEMBL3094415)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(F)c(F)c2)n1)C(O)=O Show InChI InChI=1S/C21H21ClF2N4O2S2/c1-2-3-4-5-6-16(19(29)30)32-21-26-17(22)10-18(28-21)27-20-25-15(11-31-20)12-7-8-13(23)14(24)9-12/h7-11,16H,2-6H2,1H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443824

(CHEMBL3094416)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(F)cc2F)n1)C(O)=O Show InChI InChI=1S/C21H21ClF2N4O2S2/c1-2-3-4-5-6-16(19(29)30)32-21-26-17(22)10-18(28-21)27-20-25-15(11-31-20)13-8-7-12(23)9-14(13)24/h7-11,16H,2-6H2,1H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443823

(CHEMBL3094417)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(cc2)[N+]([O-])=O)n1)C(O)=O Show InChI InChI=1S/C21H22ClN5O4S2/c1-2-3-4-5-6-16(19(28)29)33-21-24-17(22)11-18(26-21)25-20-23-15(12-32-20)13-7-9-14(10-8-13)27(30)31/h7-12,16H,2-6H2,1H3,(H,28,29)(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443815

(CHEMBL3094425)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(c(C)s2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C22H25ClN4O2S2/c1-3-4-5-9-12-16(20(28)29)31-21-24-17(23)13-18(25-21)26-22-27-19(14(2)30-22)15-10-7-6-8-11-15/h6-8,10-11,13,16H,3-5,9,12H2,1-2H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443839

(CHEMBL3094410)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc-3c(CCc4cc(OC)ccc-34)s2)n1)C(O)=O Show InChI InChI=1S/C24H27ClN4O3S2/c1-3-4-5-6-7-18(22(30)31)34-23-26-19(25)13-20(27-23)28-24-29-21-16-10-9-15(32-2)12-14(16)8-11-17(21)33-24/h9-10,12-13,18H,3-8,11H2,1-2H3,(H,30,31)(H,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443839

(CHEMBL3094410)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc-3c(CCc4cc(OC)ccc-34)s2)n1)C(O)=O Show InChI InChI=1S/C24H27ClN4O3S2/c1-3-4-5-6-7-18(22(30)31)34-23-26-19(25)13-20(27-23)28-24-29-21-16-10-9-15(32-2)12-14(16)8-11-17(21)33-24/h9-10,12-13,18H,3-8,11H2,1-2H3,(H,30,31)(H,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443832

(CHEMBL3091498)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C21H23ClN4O2S2/c1-2-3-4-8-11-16(19(27)28)30-21-24-17(22)12-18(26-21)25-20-23-15(13-29-20)14-9-6-5-7-10-14/h5-7,9-10,12-13,16H,2-4,8,11H2,1H3,(H,27,28)(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM24564
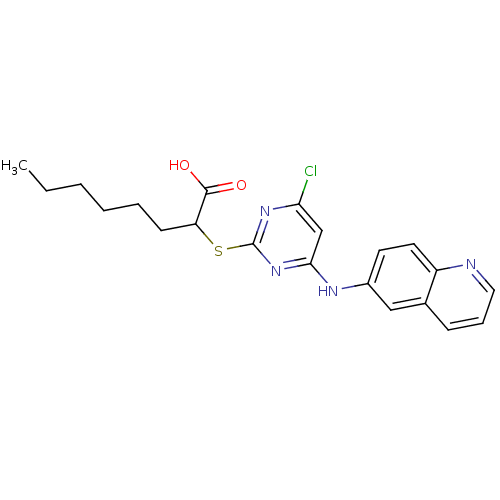
(2-{[4-chloro-6-(quinolin-6-ylamino)pyrimidin-2-yl]...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc3ncccc3c2)n1)C(O)=O Show InChI InChI=1S/C21H23ClN4O2S/c1-2-3-4-5-8-17(20(27)28)29-21-25-18(22)13-19(26-21)24-15-9-10-16-14(12-15)7-6-11-23-16/h6-7,9-13,17H,2-5,8H2,1H3,(H,27,28)(H,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PPARalpha ligand binding domain expressed in COS7 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443834

(CHEMBL3091496)Show SMILES OC(=O)CSc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1 Show InChI InChI=1S/C15H10Cl2N4O2S2/c16-9-3-1-8(2-4-9)10-6-24-14(18-10)20-12-5-11(17)19-15(21-12)25-7-13(22)23/h1-6H,7H2,(H,22,23)(H,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443822

(CHEMBL3094418)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc3CCCCc3c2)n1)C(O)=O Show InChI InChI=1S/C25H29ClN4O2S2/c1-2-3-4-5-10-20(23(31)32)34-25-28-21(26)14-22(30-25)29-24-27-19(15-33-24)18-12-11-16-8-6-7-9-17(16)13-18/h11-15,20H,2-10H2,1H3,(H,31,32)(H,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443825

(CHEMBL3094415)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(F)c(F)c2)n1)C(O)=O Show InChI InChI=1S/C21H21ClF2N4O2S2/c1-2-3-4-5-6-16(19(29)30)32-21-26-17(22)10-18(28-21)27-20-25-15(11-31-20)12-7-8-13(23)14(24)9-12/h7-11,16H,2-6H2,1H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443824

(CHEMBL3094416)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(F)cc2F)n1)C(O)=O Show InChI InChI=1S/C21H21ClF2N4O2S2/c1-2-3-4-5-6-16(19(29)30)32-21-26-17(22)10-18(28-21)27-20-25-15(11-31-20)13-8-7-12(23)9-14(13)24/h7-11,16H,2-6H2,1H3,(H,29,30)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443831

(CHEMBL3091499)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(C)cc2)n1)C(O)=O Show InChI InChI=1S/C22H25ClN4O2S2/c1-3-4-5-6-7-17(20(28)29)31-22-25-18(23)12-19(27-22)26-21-24-16(13-30-21)15-10-8-14(2)9-11-15/h8-13,17H,3-7H2,1-2H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM24564

(2-{[4-chloro-6-(quinolin-6-ylamino)pyrimidin-2-yl]...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc3ncccc3c2)n1)C(O)=O Show InChI InChI=1S/C21H23ClN4O2S/c1-2-3-4-5-8-17(20(27)28)29-21-25-18(22)13-19(26-21)24-15-9-10-16-14(12-15)7-6-11-23-16/h6-7,9-13,17H,2-5,8H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PPARgamma ligand binding domain expressed in COS7 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM24560

(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cccc(C)c2C)n1)C(O)=O Show InChI InChI=1S/C20H26ClN3O2S/c1-4-5-6-7-11-16(19(25)26)27-20-23-17(21)12-18(24-20)22-15-10-8-9-13(2)14(15)3/h8-10,12,16H,4-7,11H2,1-3H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PPARgamma ligand binding domain expressed in COS7 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443836

(CHEMBL3094433)Show SMILES CCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C17H14Cl2N4O2S2/c1-2-12(15(24)25)27-17-21-13(19)7-14(23-17)22-16-20-11(8-26-16)9-3-5-10(18)6-4-9/h3-8,12H,2H2,1H3,(H,24,25)(H,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50273682
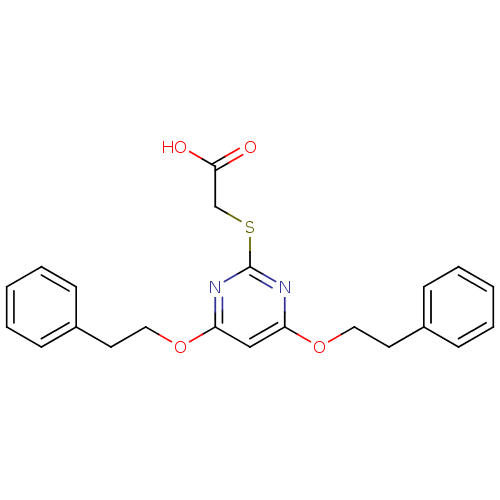
(2-(4,6-Diphenethoxypyrimidin-2-ylthio)acetic acid ...)Show InChI InChI=1S/C22H22N2O4S/c25-21(26)16-29-22-23-19(27-13-11-17-7-3-1-4-8-17)15-20(24-22)28-14-12-18-9-5-2-6-10-18/h1-10,15H,11-14,16H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PPARgamma ligand binding domain expressed in COS7 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443837

(CHEMBL3094432)Show SMILES CCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C19H18Cl2N4O2S2/c1-2-3-4-14(17(26)27)29-19-23-15(21)9-16(25-19)24-18-22-13(10-28-18)11-5-7-12(20)8-6-11/h5-10,14H,2-4H2,1H3,(H,26,27)(H,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443823

(CHEMBL3094417)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(cc2)[N+]([O-])=O)n1)C(O)=O Show InChI InChI=1S/C21H22ClN5O4S2/c1-2-3-4-5-6-16(19(28)29)33-21-24-17(22)11-18(26-21)25-20-23-15(12-32-20)13-7-9-14(10-8-13)27(30)31/h7-12,16H,2-6H2,1H3,(H,28,29)(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells microsomes assessed as reduction in PGE2 formation by RP-HPLC assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50273386
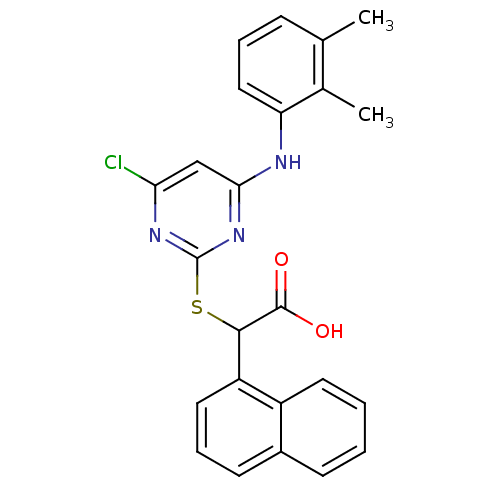
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show SMILES Cc1cccc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)c1C Show InChI InChI=1S/C24H20ClN3O2S/c1-14-7-5-12-19(15(14)2)26-21-13-20(25)27-24(28-21)31-22(23(29)30)18-11-6-9-16-8-3-4-10-17(16)18/h3-13,22H,1-2H3,(H,29,30)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PPARgamma ligand binding domain expressed in COS7 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443836

(CHEMBL3094433)Show SMILES CCC(Sc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1)C(O)=O Show InChI InChI=1S/C17H14Cl2N4O2S2/c1-2-12(15(24)25)27-17-21-13(19)7-14(23-17)22-16-20-11(8-26-16)9-3-5-10(18)6-4-9/h3-8,12H,2H2,1H3,(H,24,25)(H,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443834

(CHEMBL3091496)Show SMILES OC(=O)CSc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1 Show InChI InChI=1S/C15H10Cl2N4O2S2/c16-9-3-1-8(2-4-9)10-6-24-14(18-10)20-12-5-11(17)19-15(21-12)25-7-13(22)23/h1-6H,7H2,(H,22,23)(H,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC method |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50443834

(CHEMBL3091496)Show SMILES OC(=O)CSc1nc(Cl)cc(Nc2nc(cs2)-c2ccc(Cl)cc2)n1 Show InChI InChI=1S/C15H10Cl2N4O2S2/c16-9-3-1-8(2-4-9)10-6-24-14(18-10)20-12-5-11(17)19-15(21-12)25-7-13(22)23/h1-6H,7H2,(H,22,23)(H,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as reduction in 5-LOX product formation pre-incubated for 15 mins by HPLC based cell-free assay |
Bioorg Med Chem Lett 24: 3757-63 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.077
BindingDB Entry DOI: 10.7270/Q27W6DTR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data