

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342781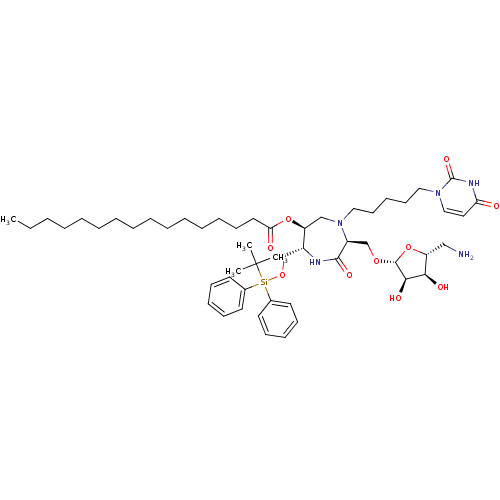 ((3S,6S,7R)-7-tert-Butyldiphenylsilyloxymethyl-4-N-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342779 ((3S,6S,7R)-7-tert-Butyldiphenylsilyloxymethyl-4-N-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342782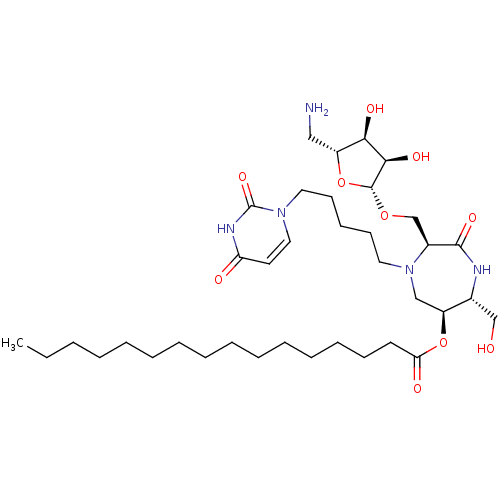 ((3S,6S,7R)-3-(5-Amino-5-deoxy-beta-D-ribos-1-yl-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342778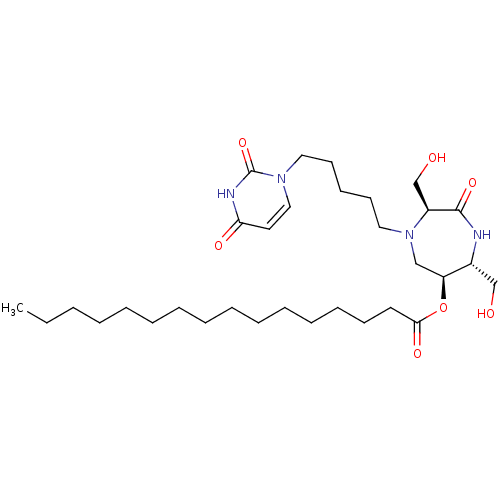 ((3S,6S,7R)-3,7-Dihydroxymethyl-6-palmitoyloxy-4-N-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342784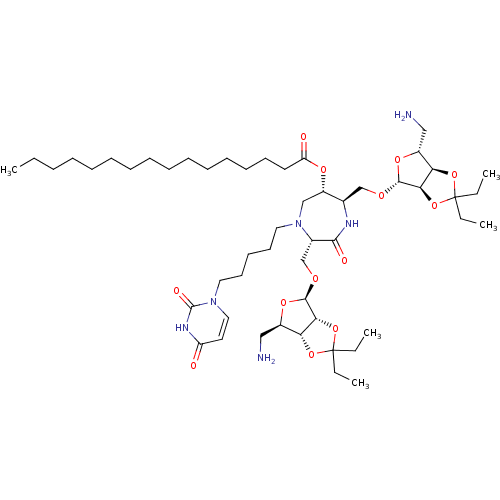 ((3S,6S,7R)-3,7-Di-(5-amino-5-deoxy-2,3-O-isopentyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342790 ((3S,6S,7R)-7-(tert-Butyldiphenylsilyloxymethyl)-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342783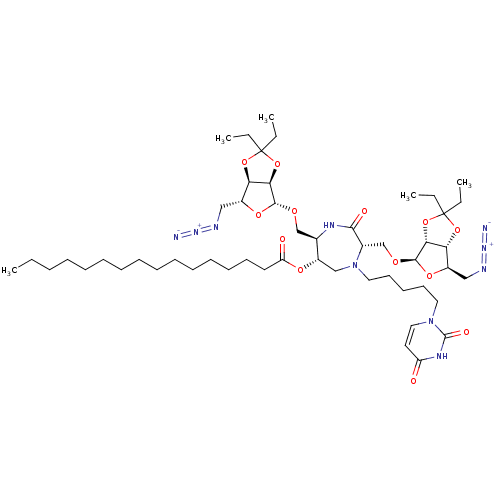 ((3S,6S,7R)-3,7-Di-(5-azido-5-deoxy-2,3-O-isopentyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Bacillus subtilis) | BDBM50321251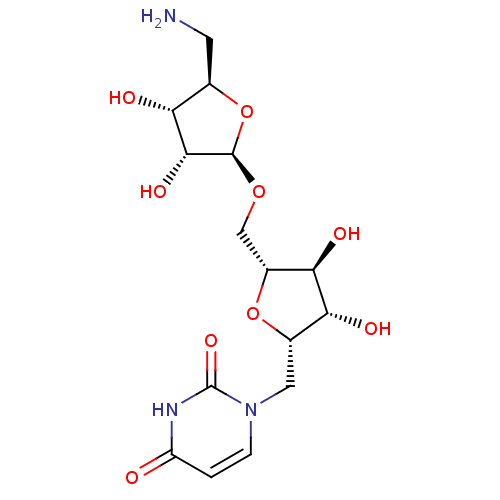 (2,5-Anhydro-1-deoxy-1-(uracil-1'-yl)-6-(5''-amino-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Bacillus subtilis transferase MraY after 30 mins | Bioorg Med Chem 18: 4560-9 (2010) Article DOI: 10.1016/j.bmc.2010.04.023 BindingDB Entry DOI: 10.7270/Q26W9B74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Bacillus subtilis) | BDBM50321253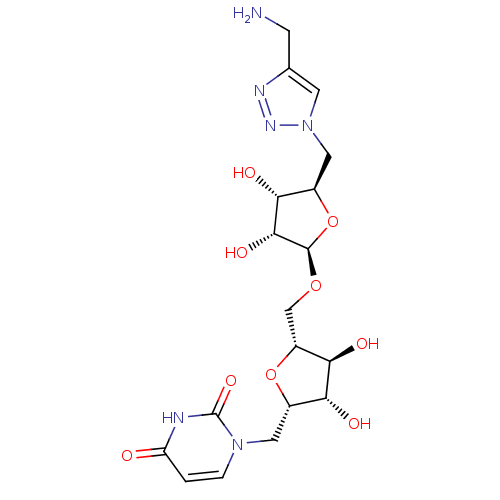 (2,5-Anhydro-3,4-dihydroxy-1-deoxy-1-(uracil-1'-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Bacillus subtilis transferase MraY after 30 mins | Bioorg Med Chem 18: 4560-9 (2010) Article DOI: 10.1016/j.bmc.2010.04.023 BindingDB Entry DOI: 10.7270/Q26W9B74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Bacillus subtilis) | BDBM50321255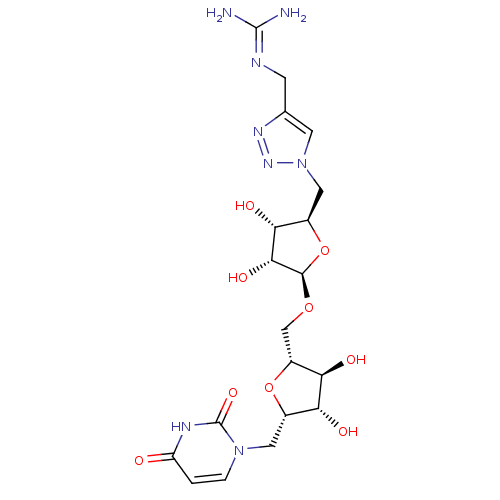 (2,5-Anhydro-3,4-dihydroxy-1-deoxy-1-(uracil-1'-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Bacillus subtilis transferase MraY after 30 mins | Bioorg Med Chem 18: 4560-9 (2010) Article DOI: 10.1016/j.bmc.2010.04.023 BindingDB Entry DOI: 10.7270/Q26W9B74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342780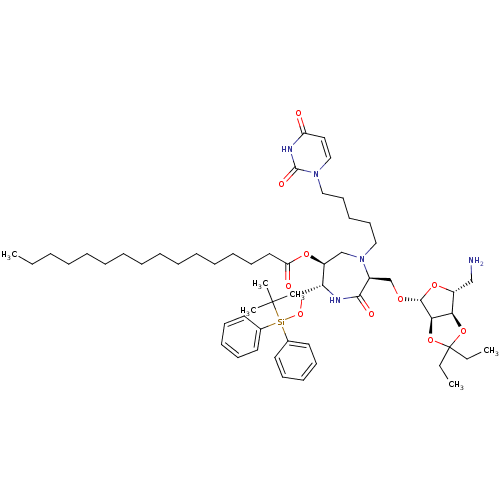 ((3S,6S,7R)-7-tert-Butyldiphenylsilyloxymethyl-4-N-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Bacillus subtilis) | BDBM50321254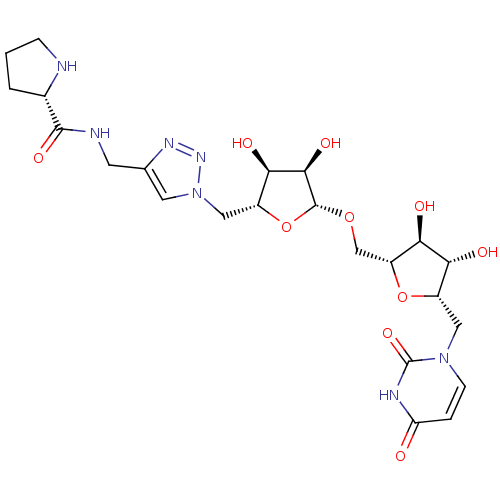 (2,5-Anhydro-3,4-dihydroxy-1-deoxy-1-(uracil-1'-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Bacillus subtilis transferase MraY after 30 mins | Bioorg Med Chem 18: 4560-9 (2010) Article DOI: 10.1016/j.bmc.2010.04.023 BindingDB Entry DOI: 10.7270/Q26W9B74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50553179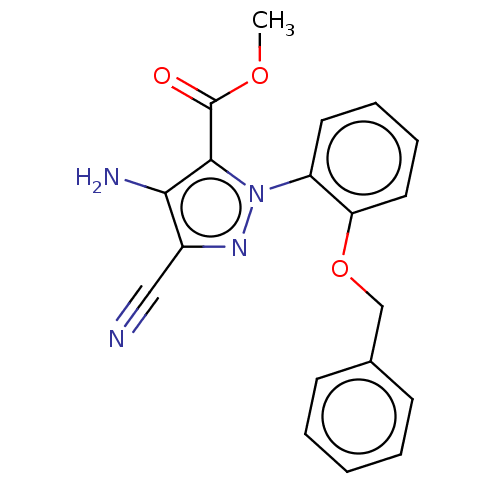 (CHEMBL4786487) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type recombinant HIV-1 subtype B reverse transcriptase p66/p51 heterodimer expressed in Escherichia coli using calf thymus DNA and... | Citation and Details Article DOI: 10.1039/d0md00025f BindingDB Entry DOI: 10.7270/Q2280C84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342789 ((3S,6S,7R)-7-tert-Butyldiphenylsilyloxymethyl-4-N-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342786 ((3S,6S,7R)-3-(Benzyloxymethyl)-7-(tertbutyldipheny...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342787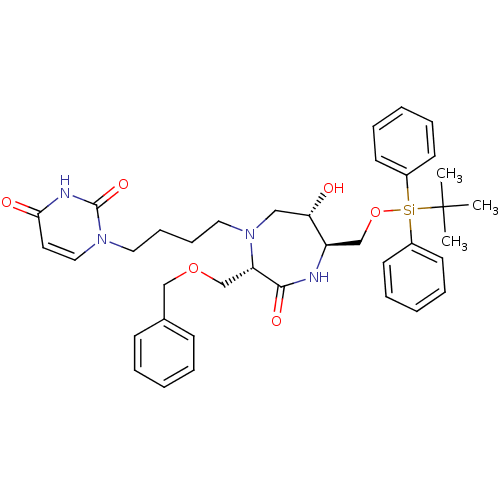 ((3S,6S,7R)-3-(Benzyloxymethyl)-7-(tertbutyldipheny...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342788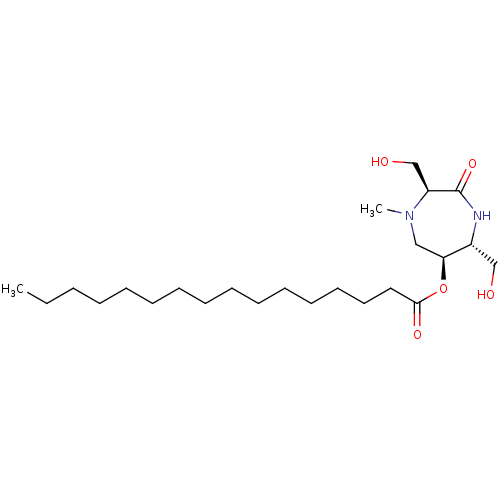 ((3S,6S,7R)-3,7-Dihydroxymethyl-4-N-methyl-6-palmit...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.74E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Bacillus subtilis) | BDBM50321252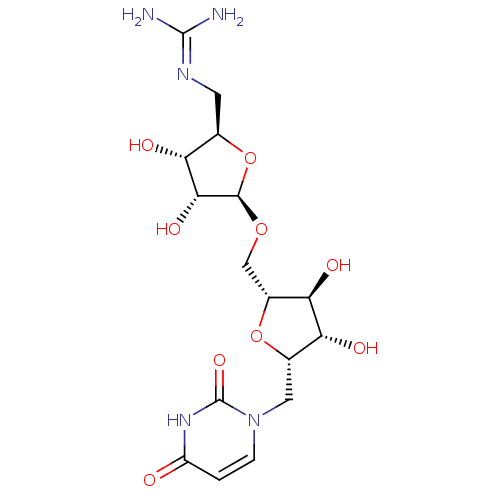 (2,5-Anhydro-3,4-dihydroxy-1-deoxy-1-(uracil-1'-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Bacillus subtilis transferase MraY after 30 mins | Bioorg Med Chem 18: 4560-9 (2010) Article DOI: 10.1016/j.bmc.2010.04.023 BindingDB Entry DOI: 10.7270/Q26W9B74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Staphylococcus aureus (strain MRSA252)) | BDBM50342785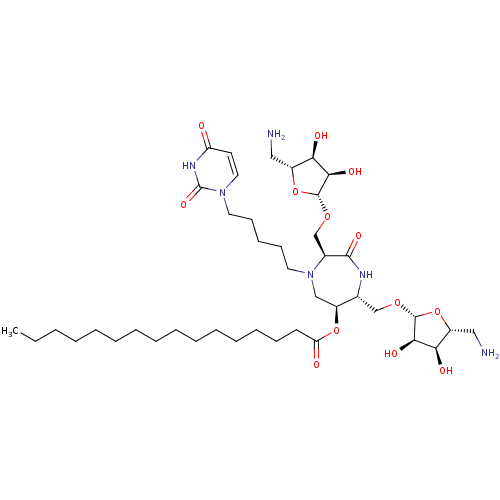 ((3S,6S,7R)-3,7-Di-(5-amino-5-deoxy-beta-D-ribos-1-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus MraY after 30 mins | Eur J Med Chem 46: 1582-92 (2011) Article DOI: 10.1016/j.ejmech.2011.02.006 BindingDB Entry DOI: 10.7270/Q2FF3SPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||