 Found 65 hits with Last Name = 'greenwood' and Initial = 'b'
Found 65 hits with Last Name = 'greenwood' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471498
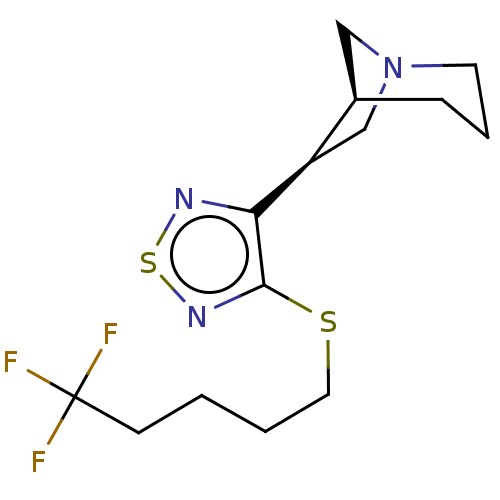
(CHEMBL150450)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C14H20F3N3S2/c15-14(16,17)5-1-2-7-21-13-12(18-22-19-13)11-9-20-6-3-4-10(11)8-20/h10-11H,1-9H2/t10-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471487
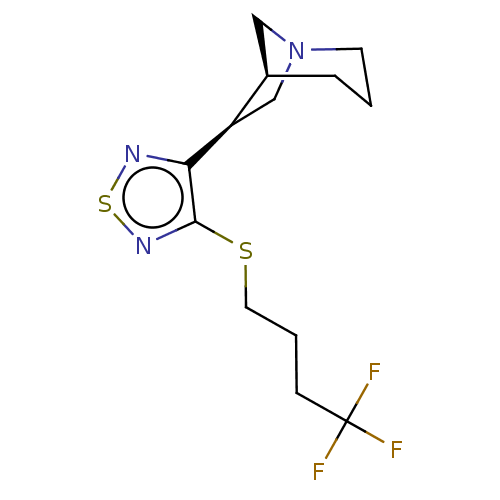
(CHEMBL436075)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471494
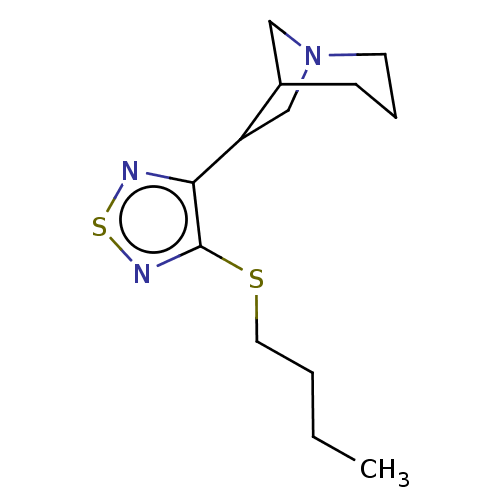
(CHEMBL153723)Show InChI InChI=1S/C13H21N3S2/c1-2-3-7-17-13-12(14-18-15-13)11-9-16-6-4-5-10(11)8-16/h10-11H,2-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471499
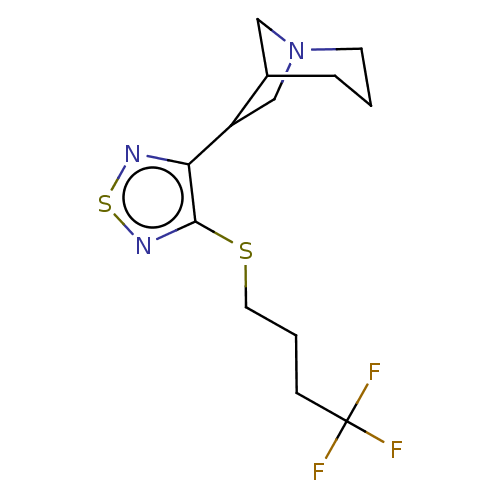
(CHEMBL345774)Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471485
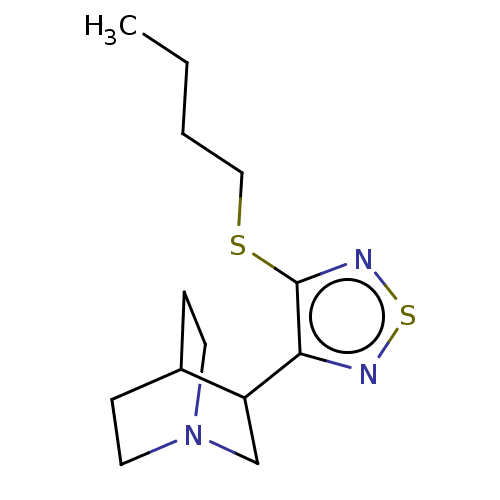
(Vedaclidine)Show SMILES CCCCSc1nsnc1C1CN2CCC1CC2 |(16.46,-2.74,;15.65,-4.05,;14.12,-3.99,;13.3,-5.3,;11.76,-5.24,;10.94,-6.55,;11.52,-7.98,;10.35,-8.98,;9.04,-8.15,;9.4,-6.67,;8.41,-5.49,;6.9,-5.77,;5.93,-4.58,;6.45,-3.12,;7.96,-2.86,;8.95,-4.05,;7.8,-3.47,;7.42,-4.98,)| Show InChI InChI=1S/C13H21N3S2/c1-2-3-8-17-13-12(14-18-15-13)11-9-16-6-4-10(11)5-7-16/h10-11H,2-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471492
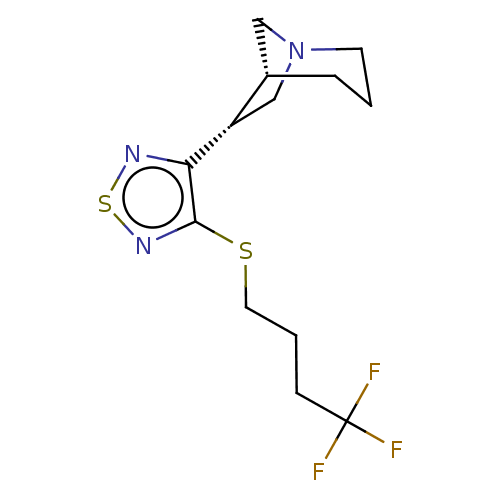
(CHEMBL150293)Show SMILES [H][C@]12CN(C[C@@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471495
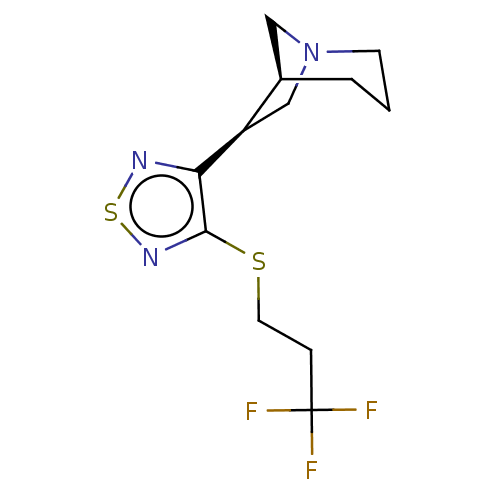
(CHEMBL153478)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCC(F)(F)F)CCC2 Show InChI InChI=1S/C12H16F3N3S2/c13-12(14,15)3-5-19-11-10(16-20-17-11)9-7-18-4-1-2-8(9)6-18/h8-9H,1-7H2/t8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471486
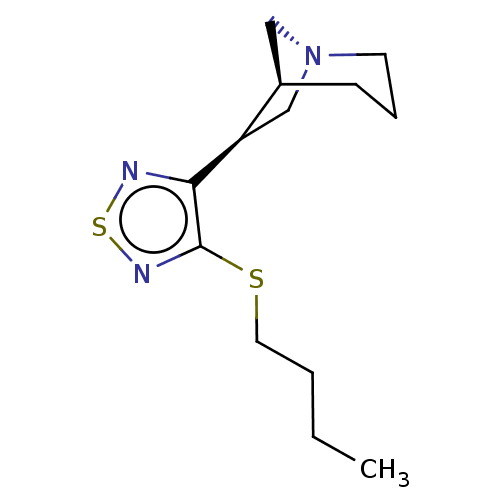
(CHEMBL343731)Show SMILES [H][C@@]12C[N@@](C[C@@]1([H])c1nsnc1SCCCC)CCC2 Show InChI InChI=1S/C13H21N3S2/c1-2-3-7-17-13-12(14-18-15-13)11-9-16-6-4-5-10(11)8-16/h10-11H,2-9H2,1H3/t10-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50033155
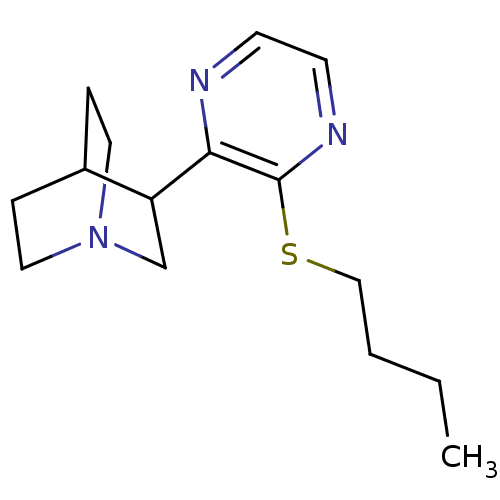
(3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...)Show SMILES CCCCSc1nccnc1C1CN2CCC1CC2 |(4.41,-8.01,;5.75,-8.78,;5.74,-10.32,;7.07,-11.09,;7.07,-12.63,;8.4,-13.4,;9.74,-12.65,;11.07,-13.42,;11.07,-14.96,;9.73,-15.71,;8.41,-14.94,;7.07,-15.71,;7.07,-17.25,;5.74,-18.01,;6.28,-16.47,;5,-16.36,;5.74,-14.93,;4.42,-15.71,;4.42,-17.25,)| Show InChI InChI=1S/C15H23N3S/c1-2-3-10-19-15-14(16-6-7-17-15)13-11-18-8-4-12(13)5-9-18/h6-7,12-13H,2-5,8-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471491
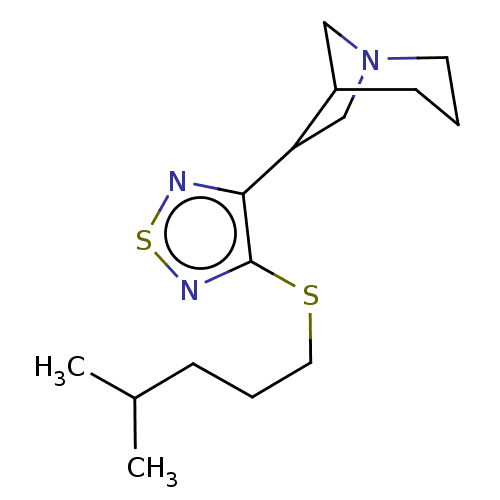
(CHEMBL357279)Show InChI InChI=1S/C15H25N3S2/c1-11(2)5-4-8-19-15-14(16-20-17-15)13-10-18-7-3-6-12(13)9-18/h11-13H,3-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
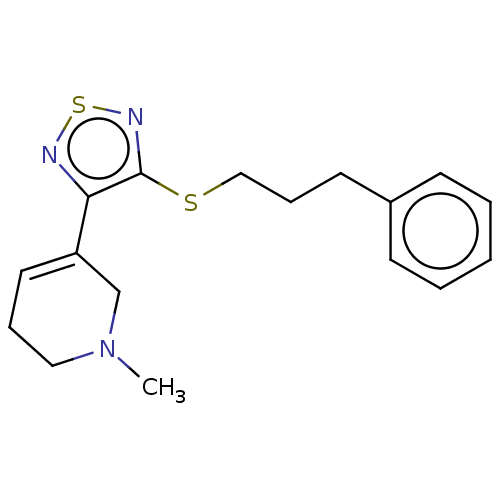
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070725
(CHEMBL318403)Show InChI InChI=1S/C28H43N5O8/c1-4-16(3)23(26(38)30-19(5-2)28(40)41)32-25(37)21-10-8-14-33(21)27(39)20(9-6-7-13-29)31-24(36)18-15-17(34)11-12-22(18)35/h11-12,15-16,19-21,23,34-35H,4-10,13-14,29H2,1-3H3,(H,30,38)(H,31,36)(H,32,37)(H,40,41)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003351
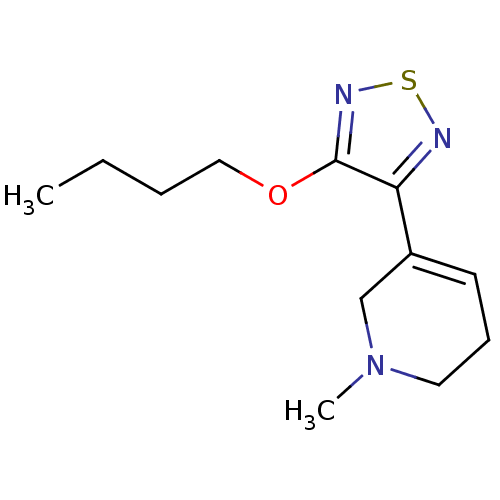
(3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...)Show InChI InChI=1S/C12H19N3OS/c1-3-4-8-16-12-11(13-17-14-12)10-6-5-7-15(2)9-10/h6H,3-5,7-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471489
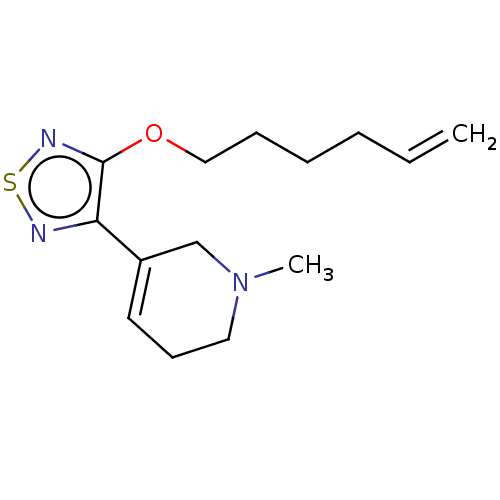
(CHEMBL153150)Show InChI InChI=1S/C14H21N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h3,8H,1,4-7,9-11H2,2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471496
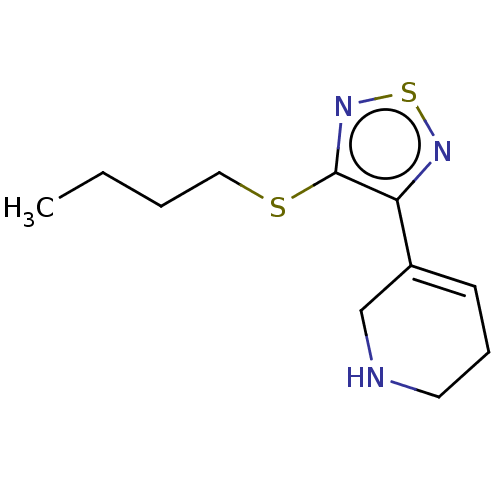
(CHEMBL153031)Show InChI InChI=1S/C11H17N3S2/c1-2-3-7-15-11-10(13-16-14-11)9-5-4-6-12-8-9/h5,12H,2-4,6-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50033151
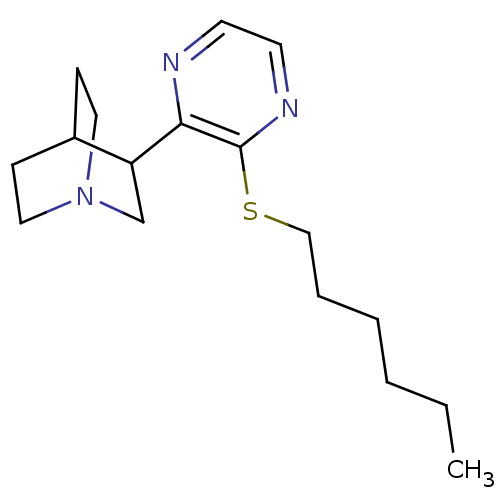
(3-(3-Hexylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...)Show SMILES CCCCCCSc1nccnc1C1CN2CCC1CC2 |(4.09,3.27,;5.44,2.49,;5.44,.95,;6.77,.18,;6.77,-1.36,;8.1,-2.13,;8.1,-3.67,;9.43,-4.44,;10.76,-3.69,;12.09,-4.46,;12.09,-6,;10.76,-6.75,;9.43,-5.98,;8.1,-6.75,;8.1,-8.29,;6.77,-9.05,;7.31,-7.51,;6.03,-7.4,;6.77,-5.97,;5.44,-6.75,;5.44,-8.29,)| Show InChI InChI=1S/C17H27N3S/c1-2-3-4-5-12-21-17-16(18-8-9-19-17)15-13-20-10-6-14(15)7-11-20/h8-9,14-15H,2-7,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471497
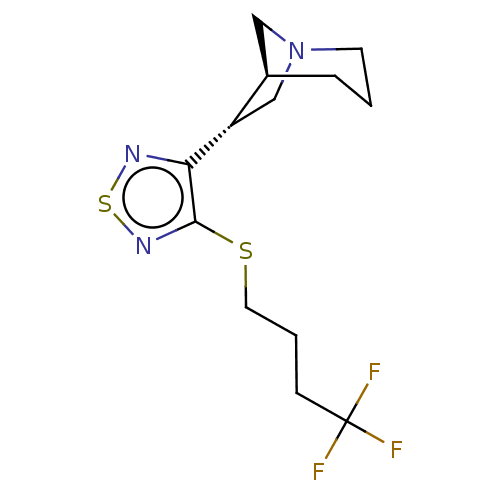
(CHEMBL150501)Show SMILES [H][C@@]12CN(C[C@@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471488
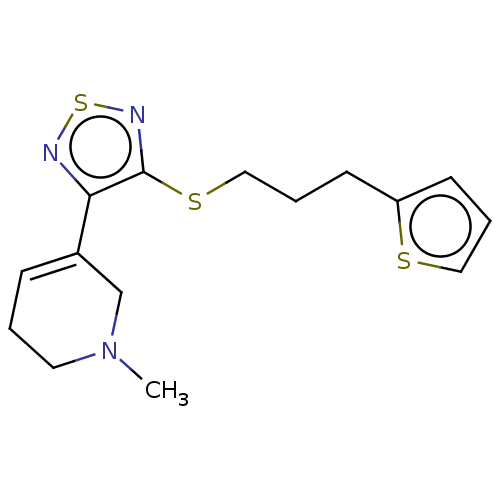
(CHEMBL152892)Show InChI InChI=1S/C15H19N3S3/c1-18-8-2-5-12(11-18)14-15(17-21-16-14)20-10-4-7-13-6-3-9-19-13/h3,5-6,9H,2,4,7-8,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471493
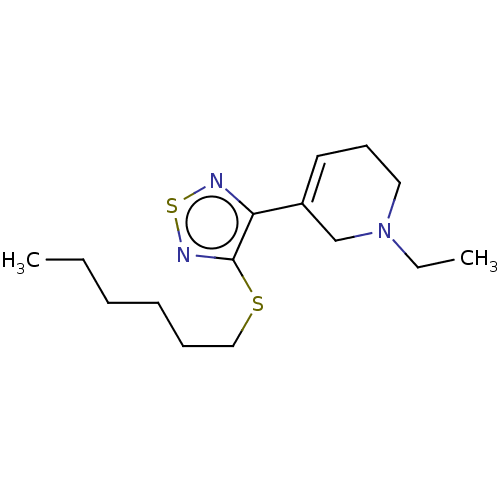
(CHEMBL152919)Show InChI InChI=1S/C15H25N3S2/c1-3-5-6-7-11-19-15-14(16-20-17-15)13-9-8-10-18(4-2)12-13/h9H,3-8,10-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471490
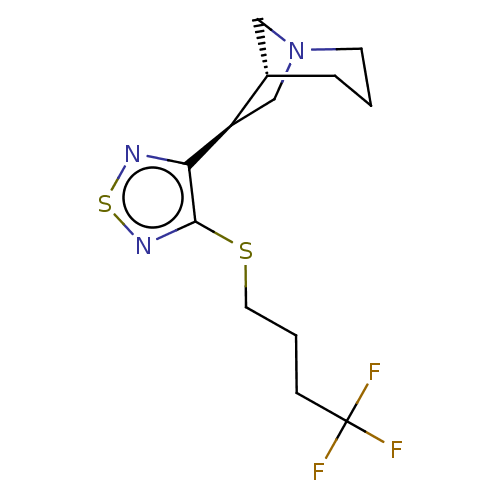
(CHEMBL154923)Show SMILES [H][C@]12CN(C[C@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458625

(CHEMBL4218013)Show SMILES CCOc1cc(c[nH]c1=O)-c1ccc(NC(=O)Nc2ccc(CN3CCN(CC)CC3)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C28H31F4N5O3/c1-3-36-9-11-37(12-10-36)17-19-5-7-21(15-22(19)28(30,31)32)34-27(39)35-24-8-6-18(13-23(24)29)20-14-25(40-4-2)26(38)33-16-20/h5-8,13-16H,3-4,9-12,17H2,1-2H3,(H,33,38)(H2,34,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458621
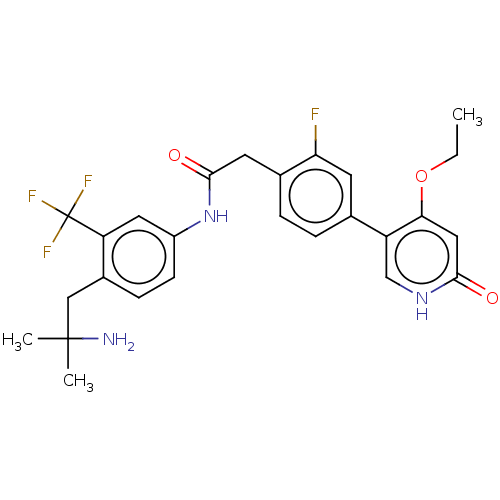
(CHEMBL4218563)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)N)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C26H27F4N3O3/c1-4-36-22-12-23(34)32-14-19(22)15-5-6-16(21(27)9-15)10-24(35)33-18-8-7-17(13-25(2,3)31)20(11-18)26(28,29)30/h5-9,11-12,14H,4,10,13,31H2,1-3H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458627
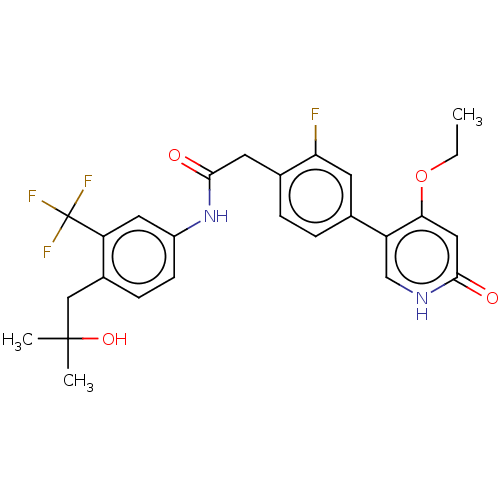
(CHEMBL4218127)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)O)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C26H26F4N2O4/c1-4-36-22-12-23(33)31-14-19(22)15-5-6-16(21(27)9-15)10-24(34)32-18-8-7-17(13-25(2,3)35)20(11-18)26(28,29)30/h5-9,11-12,14,35H,4,10,13H2,1-3H3,(H,31,33)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458628
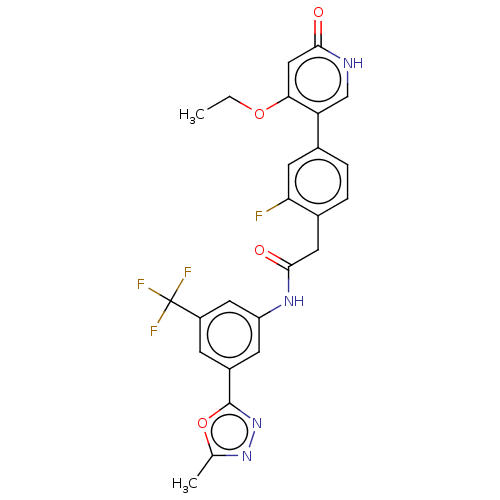
(CHEMBL4212513)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cc(cc(c2)C(F)(F)F)-c2nnc(C)o2)c(F)c1 Show InChI InChI=1S/C25H20F4N4O4/c1-3-36-21-11-22(34)30-12-19(21)14-4-5-15(20(26)8-14)9-23(35)31-18-7-16(24-33-32-13(2)37-24)6-17(10-18)25(27,28)29/h4-8,10-12H,3,9H2,1-2H3,(H,30,34)(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM347342
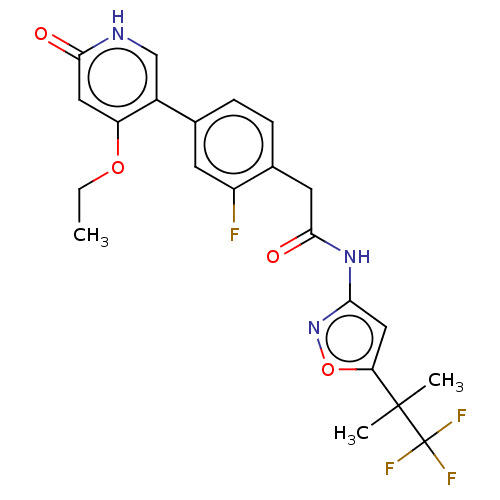
(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cc(on2)C(C)(C)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C22H21F4N3O4/c1-4-32-16-9-19(30)27-11-14(16)12-5-6-13(15(23)7-12)8-20(31)28-18-10-17(33-29-18)21(2,3)22(24,25)26/h5-7,9-11H,4,8H2,1-3H3,(H,27,30)(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM347339

(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cc(no2)C(C)(C)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C22H21F4N3O4/c1-4-32-16-9-18(30)27-11-14(16)12-5-6-13(15(23)7-12)8-19(31)28-20-10-17(29-33-20)21(2,3)22(24,25)26/h5-7,9-11H,4,8H2,1-3H3,(H,27,30)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM347313
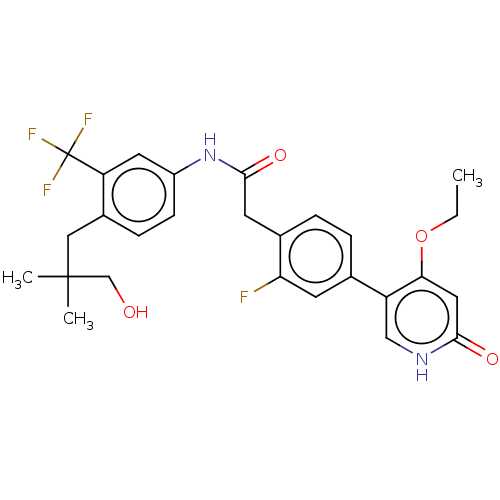
(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)CO)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C27H28F4N2O4/c1-4-37-23-12-24(35)32-14-20(23)16-5-6-17(22(28)9-16)10-25(36)33-19-8-7-18(13-26(2,3)15-34)21(11-19)27(29,30)31/h5-9,11-12,14,34H,4,10,13,15H2,1-3H3,(H,32,35)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458630
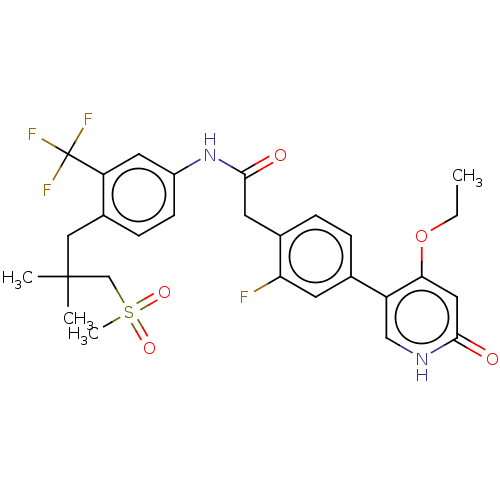
(CHEMBL4203429)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)CS(C)(=O)=O)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C28H30F4N2O5S/c1-5-39-24-13-25(35)33-15-21(24)17-6-7-18(23(29)10-17)11-26(36)34-20-9-8-19(22(12-20)28(30,31)32)14-27(2,3)16-40(4,37)38/h6-10,12-13,15H,5,11,14,16H2,1-4H3,(H,33,35)(H,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458626
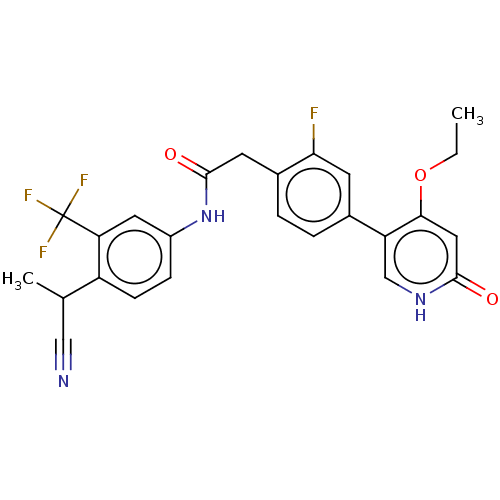
(CHEMBL4214146)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(C(C)C#N)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H21F4N3O3/c1-3-35-22-11-23(33)31-13-19(22)15-4-5-16(21(26)8-15)9-24(34)32-17-6-7-18(14(2)12-30)20(10-17)25(27,28)29/h4-8,10-11,13-14H,3,9H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458624
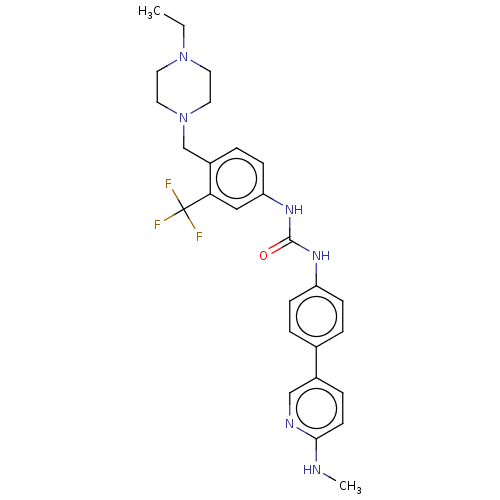
(CHEMBL4205803)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3)-c3ccc(NC)nc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C27H31F3N6O/c1-3-35-12-14-36(15-13-35)18-21-6-10-23(16-24(21)27(28,29)30)34-26(37)33-22-8-4-19(5-9-22)20-7-11-25(31-2)32-17-20/h4-11,16-17H,3,12-15,18H2,1-2H3,(H,31,32)(H2,33,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458622
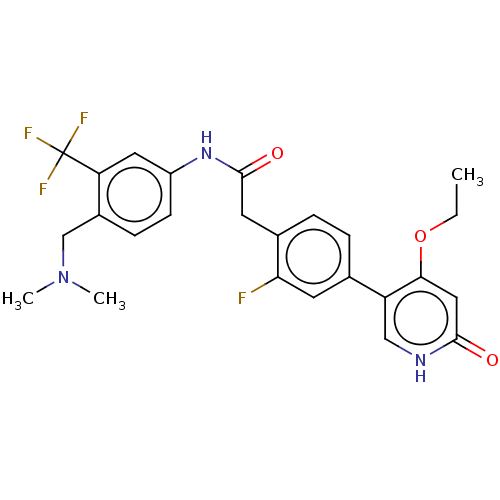
(CHEMBL4213626)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CN(C)C)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H25F4N3O3/c1-4-35-22-12-23(33)30-13-19(22)15-5-6-16(21(26)9-15)10-24(34)31-18-8-7-17(14-32(2)3)20(11-18)25(27,28)29/h5-9,11-13H,4,10,14H2,1-3H3,(H,30,33)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458629
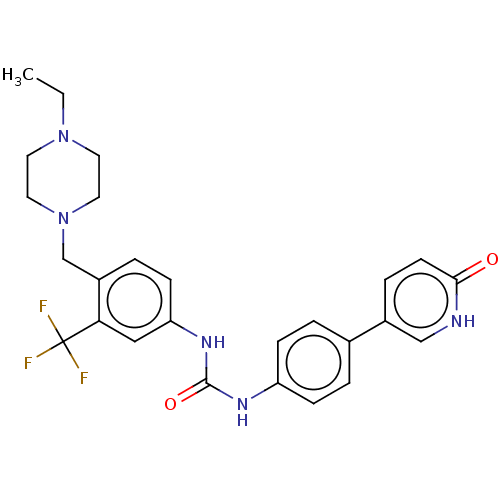
(CHEMBL4210670)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3)-c3ccc(=O)[nH]c3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H28F3N5O2/c1-2-33-11-13-34(14-12-33)17-20-5-9-22(15-23(20)26(27,28)29)32-25(36)31-21-7-3-18(4-8-21)19-6-10-24(35)30-16-19/h3-10,15-16H,2,11-14,17H2,1H3,(H,30,35)(H2,31,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM347345

(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cccc(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C22H18F4N2O3/c1-2-31-19-11-20(29)27-12-17(19)13-6-7-14(18(23)8-13)9-21(30)28-16-5-3-4-15(10-16)22(24,25)26/h3-8,10-12H,2,9H2,1H3,(H,27,29)(H,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458631
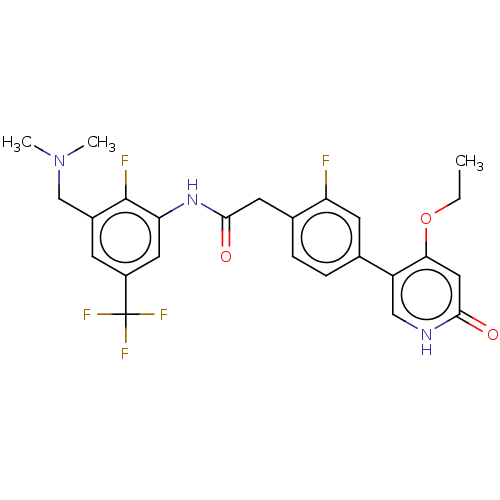
(CHEMBL4209229)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cc(cc(CN(C)C)c2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H24F5N3O3/c1-4-36-21-11-22(34)31-12-18(21)14-5-6-15(19(26)8-14)9-23(35)32-20-10-17(25(28,29)30)7-16(24(20)27)13-33(2)3/h5-8,10-12H,4,9,13H2,1-3H3,(H,31,34)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM347339

(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cc(no2)C(C)(C)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C22H21F4N3O4/c1-4-32-16-9-18(30)27-11-14(16)12-5-6-13(15(23)7-12)8-19(31)28-20-10-17(29-33-20)21(2,3)22(24,25)26/h5-7,9-11H,4,8H2,1-3H3,(H,27,30)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458630

(CHEMBL4203429)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)CS(C)(=O)=O)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C28H30F4N2O5S/c1-5-39-24-13-25(35)33-15-21(24)17-6-7-18(23(29)10-17)11-26(36)34-20-9-8-19(22(12-20)28(30,31)32)14-27(2,3)16-40(4,37)38/h6-10,12-13,15H,5,11,14,16H2,1-4H3,(H,33,35)(H,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50458624

(CHEMBL4205803)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3)-c3ccc(NC)nc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C27H31F3N6O/c1-3-35-12-14-36(15-13-35)18-21-6-10-23(16-24(21)27(28,29)30)34-26(37)33-22-8-4-19(5-9-22)20-7-11-25(31-2)32-17-20/h4-11,16-17H,3,12-15,18H2,1-2H3,(H,31,32)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458621

(CHEMBL4218563)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)N)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C26H27F4N3O3/c1-4-36-22-12-23(34)32-14-19(22)15-5-6-16(21(27)9-15)10-24(35)33-18-8-7-17(13-25(2,3)31)20(11-18)26(28,29)30/h5-9,11-12,14H,4,10,13,31H2,1-3H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458627

(CHEMBL4218127)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)O)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C26H26F4N2O4/c1-4-36-22-12-23(33)31-14-19(22)15-5-6-16(21(27)9-15)10-24(34)32-18-8-7-17(13-25(2,3)35)20(11-18)26(28,29)30/h5-9,11-12,14,35H,4,10,13H2,1-3H3,(H,31,33)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50458621

(CHEMBL4218563)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)N)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C26H27F4N3O3/c1-4-36-22-12-23(34)32-14-19(22)15-5-6-16(21(27)9-15)10-24(35)33-18-8-7-17(13-25(2,3)31)20(11-18)26(28,29)30/h5-9,11-12,14H,4,10,13,31H2,1-3H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM347313

(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)CO)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C27H28F4N2O4/c1-4-37-23-12-24(35)32-14-20(23)16-5-6-17(22(28)9-16)10-25(36)33-19-8-7-18(13-26(2,3)15-34)21(11-19)27(29,30)31/h5-9,11-12,14,34H,4,10,13,15H2,1-3H3,(H,32,35)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50458627

(CHEMBL4218127)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)O)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C26H26F4N2O4/c1-4-36-22-12-23(33)31-14-19(22)15-5-6-16(21(27)9-15)10-24(34)32-18-8-7-17(13-25(2,3)35)20(11-18)26(28,29)30/h5-9,11-12,14,35H,4,10,13H2,1-3H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM347342

(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cc(on2)C(C)(C)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C22H21F4N3O4/c1-4-32-16-9-19(30)27-11-14(16)12-5-6-13(15(23)7-12)8-20(31)28-18-10-17(33-29-18)21(2,3)22(24,25)26/h5-7,9-11H,4,8H2,1-3H3,(H,27,30)(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM347313

(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)CO)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C27H28F4N2O4/c1-4-37-23-12-24(35)32-14-20(23)16-5-6-17(22(28)9-16)10-25(36)33-19-8-7-18(13-26(2,3)15-34)21(11-19)27(29,30)31/h5-9,11-12,14,34H,4,10,13,15H2,1-3H3,(H,32,35)(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50458630

(CHEMBL4203429)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)CS(C)(=O)=O)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C28H30F4N2O5S/c1-5-39-24-13-25(35)33-15-21(24)17-6-7-18(23(29)10-17)11-26(36)34-20-9-8-19(22(12-20)28(30,31)32)14-27(2,3)16-40(4,37)38/h6-10,12-13,15H,5,11,14,16H2,1-4H3,(H,33,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50458625

(CHEMBL4218013)Show SMILES CCOc1cc(c[nH]c1=O)-c1ccc(NC(=O)Nc2ccc(CN3CCN(CC)CC3)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C28H31F4N5O3/c1-3-36-9-11-37(12-10-36)17-19-5-7-21(15-22(19)28(30,31)32)34-27(39)35-24-8-6-18(13-23(24)29)20-14-25(40-4-2)26(38)33-16-20/h5-8,13-16H,3-4,9-12,17H2,1-2H3,(H,33,38)(H2,34,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458622

(CHEMBL4213626)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CN(C)C)c(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H25F4N3O3/c1-4-35-22-12-23(33)30-13-19(22)15-5-6-16(21(26)9-15)10-24(34)31-18-8-7-17(14-32(2)3)20(11-18)25(27,28)29/h5-9,11-13H,4,10,14H2,1-3H3,(H,30,33)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458623

(CHEMBL4215099)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3)-c3ccnc(NC)c3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C27H31F3N6O/c1-3-35-12-14-36(15-13-35)18-21-6-9-23(17-24(21)27(28,29)30)34-26(37)33-22-7-4-19(5-8-22)20-10-11-32-25(16-20)31-2/h4-11,16-17H,3,12-15,18H2,1-2H3,(H,31,32)(H2,33,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50458628

(CHEMBL4212513)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cc(cc(c2)C(F)(F)F)-c2nnc(C)o2)c(F)c1 Show InChI InChI=1S/C25H20F4N4O4/c1-3-36-21-11-22(34)30-12-19(21)14-4-5-15(20(26)8-14)9-23(35)31-18-7-16(24-33-32-13(2)37-24)6-17(10-18)25(27,28)29/h4-8,10-12H,3,9H2,1-2H3,(H,30,34)(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM347339

(2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...)Show SMILES CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2cc(no2)C(C)(C)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C22H21F4N3O4/c1-4-32-16-9-18(30)27-11-14(16)12-5-6-13(15(23)7-12)8-19(31)28-20-10-17(29-33-20)21(2,3)22(24,25)26/h5-7,9-11H,4,8H2,1-3H3,(H,27,30)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... |
ACS Med Chem Lett 9: 623-628 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00035
BindingDB Entry DOI: 10.7270/Q2TB19GW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data