 Found 202 hits with Last Name = 'himmelsbach' and Initial = 'rj'
Found 202 hits with Last Name = 'himmelsbach' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50030815
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005417
(CHEMBL266334 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...)Show SMILES CCSC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C31H52N4O7S2/c1-4-43-31(30(39)32-25(20-23-11-7-5-8-12-23)28(37)27(36)19-22(2)3)33-29(38)26(21-24-13-9-6-10-14-24)34-44(40,41)35-15-17-42-18-16-35/h6,9-10,13-14,22-23,25-28,31,34,36-37H,4-5,7-8,11-12,15-21H2,1-3H3,(H,32,39)(H,33,38)/t25-,26-,27-,28+,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005437
(CHEMBL8477 | N-[Allylsulfanyl-(1-cyclohexylmethyl-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)SCC=C Show InChI InChI=1S/C32H52N4O7S2/c1-4-19-44-32(31(40)33-26(21-24-11-7-5-8-12-24)29(38)28(37)20-23(2)3)34-30(39)27(22-25-13-9-6-10-14-25)35-45(41,42)36-15-17-43-18-16-36/h4,6,9-10,13-14,23-24,26-29,32,35,37-38H,1,5,7-8,11-12,15-22H2,2-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29+,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012250

(CHEMBL58947 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COC(=O)CC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)CC(C)C Show InChI InChI=1S/C32H52N4O9S/c1-22(2)18-28(37)30(39)25(19-23-10-6-4-7-11-23)33-31(40)26(21-29(38)44-3)34-32(41)27(20-24-12-8-5-9-13-24)35-46(42,43)36-14-16-45-17-15-36/h5,8-9,12-13,22-23,25-28,30,35,37,39H,4,6-7,10-11,14-21H2,1-3H3,(H,33,40)(H,34,41)/t25-,26?,27-,28+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005443

(CHEMBL441325 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)OCC(F)(F)F Show InChI InChI=1S/C31H49F3N4O8S/c1-21(2)17-26(39)27(40)24(18-22-9-5-3-6-10-22)35-29(42)30(46-20-31(32,33)34)36-28(41)25(19-23-11-7-4-8-12-23)37-47(43,44)38-13-15-45-16-14-38/h4,7-8,11-12,21-22,24-27,30,37,39-40H,3,5-6,9-10,13-20H2,1-2H3,(H,35,42)(H,36,41)/t24-,25-,26-,27+,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012246

(CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)CC(C)C Show InChI InChI=1S/C31H50N4O9S/c1-21(2)18-26(36)28(37)24(19-22-10-6-4-7-11-22)32-30(39)27(31(40)43-3)33-29(38)25(20-23-12-8-5-9-13-23)34-45(41,42)35-14-16-44-17-15-35/h5,8-9,12-13,21-22,24-28,34,36-37H,4,6-7,10-11,14-20H2,1-3H3,(H,32,39)(H,33,38)/t24-,25-,26+,27?,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012246

(CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)CC(C)C Show InChI InChI=1S/C31H50N4O9S/c1-21(2)18-26(36)28(37)24(19-22-10-6-4-7-11-22)32-30(39)27(31(40)43-3)33-29(38)25(20-23-12-8-5-9-13-23)34-45(41,42)35-14-16-44-17-15-35/h5,8-9,12-13,21-22,24-28,34,36-37H,4,6-7,10-11,14-20H2,1-3H3,(H,32,39)(H,33,38)/t24-,25-,26+,27?,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50282993
((2S,4R)-4-Cyclohexyl-2-hydroxy-3-((R)-3-methylsulf...)Show SMILES CSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C(=O)OC(C)C Show InChI InChI=1S/C31H48N4O7S/c1-21(2)42-30(39)27(36)24(18-22-10-6-4-7-11-22)32-29(38)26(20-43-3)33-28(37)25(19-23-12-8-5-9-13-23)34-31(40)35-14-16-41-17-15-35/h5,8-9,12-13,21-22,24-27,36H,4,6-7,10-11,14-20H2,1-3H3,(H,32,38)(H,33,37)(H,34,40)/t24-,25-,26-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005431

(CHEMBL8665 | N-[(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES CCCOC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C32H54N4O8S/c1-4-17-44-32(31(40)33-26(21-24-11-7-5-8-12-24)29(38)28(37)20-23(2)3)34-30(39)27(22-25-13-9-6-10-14-25)35-45(41,42)36-15-18-43-19-16-36/h6,9-10,13-14,23-24,26-29,32,35,37-38H,4-5,7-8,11-12,15-22H2,1-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29+,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005442

(CHEMBL262712 | N-[Allyloxy-(1-cyclohexylmethyl-2,3...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)OCC=C Show InChI InChI=1S/C32H52N4O8S/c1-4-17-44-32(31(40)33-26(21-24-11-7-5-8-12-24)29(38)28(37)20-23(2)3)34-30(39)27(22-25-13-9-6-10-14-25)35-45(41,42)36-15-18-43-19-16-36/h4,6,9-10,13-14,23-24,26-29,32,35,37-38H,1,5,7-8,11-12,15-22H2,2-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29+,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005445
(CHEMBL267277 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N6O7S/c1-23(2)17-30(40)31(41)27(18-24-9-5-3-6-10-24)36-32(42)28(20-26-21-34-22-35-26)37-33(43)29(19-25-11-7-4-8-12-25)38-47(44,45)39-13-15-46-16-14-39/h4,7-8,11-12,21-24,27-31,38,40-41H,3,5-6,9-10,13-20H2,1-2H3,(H,34,35)(H,36,42)(H,37,43)/t27-,28?,29-,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012251

(2-{2-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...)Show SMILES CC(C)C[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)NC(Cc1cnc[nH]1)C(O)=O Show InChI InChI=1S/C36H55N7O10S/c1-23(2)17-30(44)32(45)27(18-24-9-5-3-6-10-24)39-34(47)31(35(48)40-29(36(49)50)20-26-21-37-22-38-26)41-33(46)28(19-25-11-7-4-8-12-25)42-54(51,52)43-13-15-53-16-14-43/h4,7-8,11-12,21-24,27-32,42,44-45H,3,5-6,9-10,13-20H2,1-2H3,(H,37,38)(H,39,47)(H,40,48)(H,41,46)(H,49,50)/t27-,28-,29?,30+,31?,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005428
(CHEMBL8836 | N-[(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES CCOC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C31H52N4O8S/c1-4-43-31(30(39)32-25(20-23-11-7-5-8-12-23)28(37)27(36)19-22(2)3)33-29(38)26(21-24-13-9-6-10-14-24)34-44(40,41)35-15-17-42-18-16-35/h6,9-10,13-14,22-23,25-28,31,34,36-37H,4-5,7-8,11-12,15-21H2,1-3H3,(H,32,39)(H,33,38)/t25-,26-,27-,28+,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012246

(CHEMBL58153 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)CC(C)C Show InChI InChI=1S/C31H50N4O9S/c1-21(2)18-26(36)28(37)24(19-22-10-6-4-7-11-22)32-30(39)27(31(40)43-3)33-29(38)25(20-23-12-8-5-9-13-23)34-45(41,42)35-14-16-44-17-15-35/h5,8-9,12-13,21-22,24-28,34,36-37H,4,6-7,10-11,14-20H2,1-3H3,(H,32,39)(H,33,38)/t24-,25-,26+,27?,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50030815

(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005422

(5-Cyclohexyl-2,2-difluoro-3-hydroxy-4-{2-[2-(morph...)Show SMILES CCCOC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C(F)(F)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C35H56F2N6O9S/c1-2-19-52-33(40-31(45)29(25-27-11-7-4-8-12-27)41-53(48,49)43-17-22-51-23-18-43)32(46)39-28(24-26-9-5-3-6-10-26)30(44)35(36,37)34(47)38-13-14-42-15-20-50-21-16-42/h4,7-8,11-12,26,28-30,33,41,44H,2-3,5-6,9-10,13-25H2,1H3,(H,38,47)(H,39,46)(H,40,45)/t28-,29-,30+,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005423

(CHEMBL266139 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)OCC#C Show InChI InChI=1S/C32H50N4O8S/c1-4-17-44-32(31(40)33-26(21-24-11-7-5-8-12-24)29(38)28(37)20-23(2)3)34-30(39)27(22-25-13-9-6-10-14-25)35-45(41,42)36-15-18-43-19-16-36/h1,6,9-10,13-14,23-24,26-29,32,35,37-38H,5,7-8,11-12,15-22H2,2-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29+,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012249
(CHEMBL58256 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES CC(C)C[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)OC(C)C Show InChI InChI=1S/C33H54N4O9S/c1-22(2)19-28(38)30(39)26(20-24-11-7-5-8-12-24)34-32(41)29(33(42)46-23(3)4)35-31(40)27(21-25-13-9-6-10-14-25)36-47(43,44)37-15-17-45-18-16-37/h6,9-10,13-14,22-24,26-30,36,38-39H,5,7-8,11-12,15-21H2,1-4H3,(H,34,41)(H,35,40)/t26-,27-,28+,29?,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006202

(3-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COc1ccc(C[C@H](NC(=O)CC(C)(C)N)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)[C@@H](O)CC(C)C)cc1 Show InChI InChI=1S/C35H56N6O6/c1-22(2)15-30(42)32(44)27(16-23-9-7-6-8-10-23)40-34(46)29(18-25-20-37-21-38-25)41-33(45)28(39-31(43)19-35(3,4)36)17-24-11-13-26(47-5)14-12-24/h11-14,20-23,27-30,32,42,44H,6-10,15-19,36H2,1-5H3,(H,37,38)(H,39,43)(H,40,46)(H,41,45)/t27-,28-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012257

(CHEMBL299252 | N-[1-Cyclohexylmethyl-3,3-difluoro-...)Show SMILES COC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)C(=O)C(F)(F)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C34H50F2N6O10S/c1-50-32(46)28(39-30(44)27(23-25-10-6-3-7-11-25)40-53(48,49)42-16-20-52-21-17-42)31(45)38-26(22-24-8-4-2-5-9-24)29(43)34(35,36)33(47)37-12-13-41-14-18-51-19-15-41/h3,6-7,10-11,24,26-28,40H,2,4-5,8-9,12-23H2,1H3,(H,37,47)(H,38,45)(H,39,44)/t26-,27-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17941
((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30-,32+,33+,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50012252

(CHEMBL57492 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES CC(C)C[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)OCC=C Show InChI InChI=1S/C33H52N4O9S/c1-4-17-46-33(42)29(32(41)34-26(21-24-11-7-5-8-12-24)30(39)28(38)20-23(2)3)35-31(40)27(22-25-13-9-6-10-14-25)36-47(43,44)37-15-18-45-19-16-37/h4,6,9-10,13-14,23-24,26-30,36,38-39H,1,5,7-8,11-12,15-22H2,2-3H3,(H,34,41)(H,35,40)/t26-,27-,28+,29?,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005439

((1-{[(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...)Show SMILES CCSC(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C32H53N3O6S/c1-7-42-30(29(39)33-24(19-22-14-10-8-11-15-22)27(37)26(36)18-21(2)3)35-28(38)25(20-23-16-12-9-13-17-23)34-31(40)41-32(4,5)6/h9,12-13,16-17,21-22,24-27,30,36-37H,7-8,10-11,14-15,18-20H2,1-6H3,(H,33,39)(H,34,40)(H,35,38)/t24-,25-,26-,27+,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012247

(4-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-hexyl...)Show SMILES COC(=O)CCC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)CC(C)C Show InChI InChI=1S/C33H54N4O9S/c1-23(2)20-29(38)31(40)27(21-24-10-6-4-7-11-24)35-32(41)26(14-15-30(39)45-3)34-33(42)28(22-25-12-8-5-9-13-25)36-47(43,44)37-16-18-46-19-17-37/h5,8-9,12-13,23-24,26-29,31,36,38,40H,4,6-7,10-11,14-22H2,1-3H3,(H,34,42)(H,35,41)/t26?,27-,28-,29+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005436

(4-{2-Allylsulfanyl-2-[2-(morpholine-4-sulfonylamin...)Show SMILES O[C@@H](CC(=O)NCCN1CCOCC1)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)SCC=C Show InChI InChI=1S/C35H56N6O8S2/c1-2-23-50-35(38-33(44)30(25-28-11-7-4-8-12-28)39-51(46,47)41-17-21-49-22-18-41)34(45)37-29(24-27-9-5-3-6-10-27)31(42)26-32(43)36-13-14-40-15-19-48-20-16-40/h2,4,7-8,11-12,27,29-31,35,39,42H,1,3,5-6,9-10,13-26H2,(H,36,43)(H,37,45)(H,38,44)/t29-,30-,31-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228894

(CHEMBL312754)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |wU:57.60,4.4,44.44,24.25,wD:20.21,8.8,61.64,2.2,(10.41,1.37,;9.34,.76,;9.34,-.78,;10.41,-1.4,;8.01,-1.55,;6.67,-.78,;5.34,-1.55,;5.33,-2.78,;4,-.77,;2.67,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.34,.77,;-2.41,1.39,;-1.34,-.77,;,-1.54,;4.01,.77,;5.35,1.53,;6.41,.91,;5.35,3.07,;6.69,3.84,;8.02,3.06,;8.02,1.83,;9.36,3.83,;10.7,3.06,;12.03,3.82,;13.37,3.05,;14.71,3.81,;16.04,3.04,;17.11,3.65,;16.03,1.81,;9.36,5.37,;8.03,6.14,;7.14,5.63,;8.03,7.68,;6.7,8.46,;6.7,9.48,;4.02,3.85,;2.95,3.24,;4.03,5.08,;8,-3.09,;6.94,-3.71,;9.34,-3.87,;9.33,-5.41,;10.67,-6.18,;12,-5.42,;13.4,-6.06,;14.43,-4.91,;13.66,-3.58,;12.15,-3.89,;8,-6.18,;7.11,-5.67,;8,-7.72,;9.25,-8.61,;8.77,-10.07,;7.23,-10.07,;6.76,-8.61,;5.3,-8.12,;5.05,-6.92,;4.14,-9.14,;2.68,-8.65,;2.44,-7.45,;1.52,-9.68,;1.73,-10.68,;.36,-9.29,)| Show InChI InChI=1S/C43H67N13O10/c1-7-24(4)35(40(63)53-31(19-27-20-47-22-49-27)41(64)56-17-9-11-32(56)38(61)50-25(5)42(65)66)55-37(60)30(18-26-12-14-28(57)15-13-26)52-39(62)34(23(2)3)54-36(59)29(51-33(58)21-46-6)10-8-16-48-43(44)45/h12-15,20,22-25,29-32,34-35,46,57H,7-11,16-19,21H2,1-6H3,(H,47,49)(H,50,61)(H,51,58)(H,52,62)(H,53,63)(H,54,59)(H,55,60)(H,65,66)(H4,44,45,48)/t24-,25-,29-,30-,31-,32-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005433
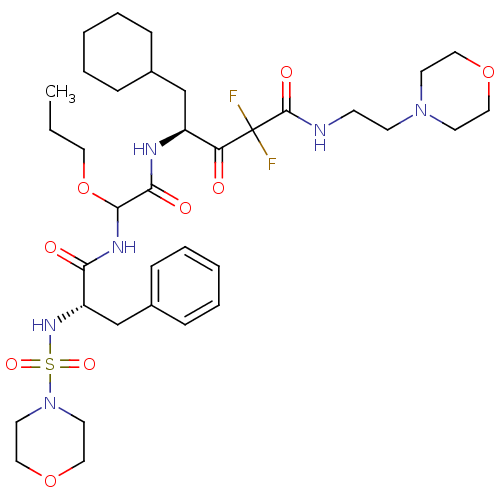
(5-Cyclohexyl-2,2-difluoro-4-{2-[2-(morpholine-4-su...)Show SMILES CCCOC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)C(=O)C(F)(F)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C35H54F2N6O9S/c1-2-19-52-33(40-31(45)29(25-27-11-7-4-8-12-27)41-53(48,49)43-17-22-51-23-18-43)32(46)39-28(24-26-9-5-3-6-10-26)30(44)35(36,37)34(47)38-13-14-42-15-20-50-21-16-42/h4,7-8,11-12,26,28-29,33,41H,2-3,5-6,9-10,13-25H2,1H3,(H,38,47)(H,39,46)(H,40,45)/t28-,29-,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012255
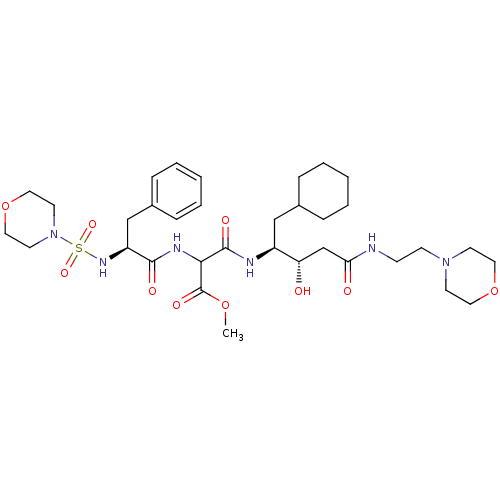
(CHEMBL293328 | N-[1-Cyclohexylmethyl-2-hydroxy-3-(...)Show SMILES COC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)CC(=O)NCCN1CCOCC1 Show InChI InChI=1S/C34H54N6O10S/c1-48-34(45)31(37-32(43)28(23-26-10-6-3-7-11-26)38-51(46,47)40-16-20-50-21-17-40)33(44)36-27(22-25-8-4-2-5-9-25)29(41)24-30(42)35-12-13-39-14-18-49-19-15-39/h3,6-7,10-11,25,27-29,31,38,41H,2,4-5,8-9,12-24H2,1H3,(H,35,42)(H,36,44)(H,37,43)/t27-,28-,29-,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005420
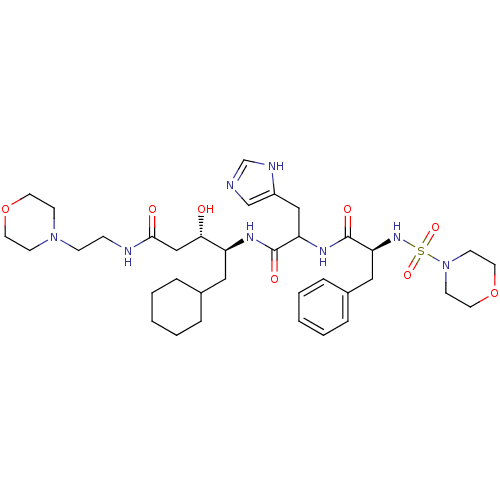
(5-Cyclohexyl-3-hydroxy-4-{3-(1H-imidazol-4-yl)-2-[...)Show SMILES O[C@@H](CC(=O)NCCN1CCOCC1)[C@H](CC1CCCCC1)NC(=O)C(Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C36H56N8O8S/c45-33(24-34(46)38-11-12-43-13-17-51-18-14-43)30(21-27-7-3-1-4-8-27)40-35(47)31(23-29-25-37-26-39-29)41-36(48)32(22-28-9-5-2-6-10-28)42-53(49,50)44-15-19-52-20-16-44/h2,5-6,9-10,25-27,30-33,42,45H,1,3-4,7-8,11-24H2,(H,37,39)(H,38,46)(H,40,47)(H,41,48)/t30-,31?,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228894

(CHEMBL312754)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |wU:57.60,4.4,44.44,24.25,wD:20.21,8.8,61.64,2.2,(10.41,1.37,;9.34,.76,;9.34,-.78,;10.41,-1.4,;8.01,-1.55,;6.67,-.78,;5.34,-1.55,;5.33,-2.78,;4,-.77,;2.67,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.34,.77,;-2.41,1.39,;-1.34,-.77,;,-1.54,;4.01,.77,;5.35,1.53,;6.41,.91,;5.35,3.07,;6.69,3.84,;8.02,3.06,;8.02,1.83,;9.36,3.83,;10.7,3.06,;12.03,3.82,;13.37,3.05,;14.71,3.81,;16.04,3.04,;17.11,3.65,;16.03,1.81,;9.36,5.37,;8.03,6.14,;7.14,5.63,;8.03,7.68,;6.7,8.46,;6.7,9.48,;4.02,3.85,;2.95,3.24,;4.03,5.08,;8,-3.09,;6.94,-3.71,;9.34,-3.87,;9.33,-5.41,;10.67,-6.18,;12,-5.42,;13.4,-6.06,;14.43,-4.91,;13.66,-3.58,;12.15,-3.89,;8,-6.18,;7.11,-5.67,;8,-7.72,;9.25,-8.61,;8.77,-10.07,;7.23,-10.07,;6.76,-8.61,;5.3,-8.12,;5.05,-6.92,;4.14,-9.14,;2.68,-8.65,;2.44,-7.45,;1.52,-9.68,;1.73,-10.68,;.36,-9.29,)| Show InChI InChI=1S/C43H67N13O10/c1-7-24(4)35(40(63)53-31(19-27-20-47-22-49-27)41(64)56-17-9-11-32(56)38(61)50-25(5)42(65)66)55-37(60)30(18-26-12-14-28(57)15-13-26)52-39(62)34(23(2)3)54-36(59)29(51-33(58)21-46-6)10-8-16-48-43(44)45/h12-15,20,22-25,29-32,34-35,46,57H,7-11,16-19,21H2,1-6H3,(H,47,49)(H,50,61)(H,51,58)(H,52,62)(H,53,63)(H,54,59)(H,55,60)(H,65,66)(H4,44,45,48)/t24-,25-,29-,30-,31-,32-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005418

(5-Cyclohexyl-4-{2-ethoxy-2-[2-(morpholine-4-sulfon...)Show SMILES CCOC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)CC(=O)NCCN1CCOCC1 Show InChI InChI=1S/C34H56N6O9S/c1-2-49-34(37-32(43)29(24-27-11-7-4-8-12-27)38-50(45,46)40-17-21-48-22-18-40)33(44)36-28(23-26-9-5-3-6-10-26)30(41)25-31(42)35-13-14-39-15-19-47-20-16-39/h4,7-8,11-12,26,28-30,34,38,41H,2-3,5-6,9-10,13-25H2,1H3,(H,35,42)(H,36,44)(H,37,43)/t28-,29-,30-,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50005436

(4-{2-Allylsulfanyl-2-[2-(morpholine-4-sulfonylamin...)Show SMILES O[C@@H](CC(=O)NCCN1CCOCC1)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)SCC=C Show InChI InChI=1S/C35H56N6O8S2/c1-2-23-50-35(38-33(44)30(25-28-11-7-4-8-12-28)39-51(46,47)41-17-21-49-22-18-41)34(45)37-29(24-27-9-5-3-6-10-27)31(42)26-32(43)36-13-14-40-15-19-48-20-16-40/h2,4,7-8,11-12,27,29-31,35,39,42H,1,3,5-6,9-10,13-26H2,(H,36,43)(H,37,45)(H,38,44)/t29-,30-,31-,35?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine cathepsin D |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50005417

(CHEMBL266334 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...)Show SMILES CCSC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C31H52N4O7S2/c1-4-43-31(30(39)32-25(20-23-11-7-5-8-12-23)28(37)27(36)19-22(2)3)33-29(38)26(21-24-13-9-6-10-14-24)34-44(40,41)35-15-17-42-18-16-35/h6,9-10,13-14,22-23,25-28,31,34,36-37H,4-5,7-8,11-12,15-21H2,1-3H3,(H,32,39)(H,33,38)/t25-,26-,27-,28+,31?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine cathepsin D |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50005442

(CHEMBL262712 | N-[Allyloxy-(1-cyclohexylmethyl-2,3...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)OCC=C Show InChI InChI=1S/C32H52N4O8S/c1-4-17-44-32(31(40)33-26(21-24-11-7-5-8-12-24)29(38)28(37)20-23(2)3)34-30(39)27(22-25-13-9-6-10-14-25)35-45(41,42)36-15-18-43-19-16-36/h4,6,9-10,13-14,23-24,26-29,32,35,37-38H,1,5,7-8,11-12,15-22H2,2-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine cathepsin D |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005444
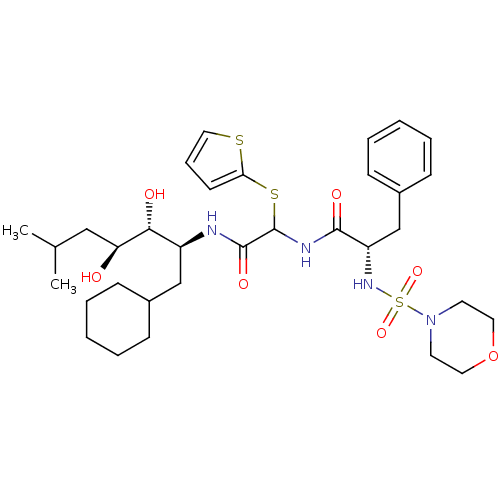
(CHEMBL406692 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)Sc1cccs1 Show InChI InChI=1S/C33H50N4O7S3/c1-23(2)20-28(38)30(39)26(21-24-10-5-3-6-11-24)34-32(41)33(46-29-14-9-19-45-29)35-31(40)27(22-25-12-7-4-8-13-25)36-47(42,43)37-15-17-44-18-16-37/h4,7-9,12-14,19,23-24,26-28,30,33,36,38-39H,3,5-6,10-11,15-18,20-22H2,1-2H3,(H,34,41)(H,35,40)/t26-,27-,28-,30+,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50005428

(CHEMBL8836 | N-[(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES CCOC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C31H52N4O8S/c1-4-43-31(30(39)32-25(20-23-11-7-5-8-12-23)28(37)27(36)19-22(2)3)33-29(38)26(21-24-13-9-6-10-14-24)34-44(40,41)35-15-17-42-18-16-35/h6,9-10,13-14,22-23,25-28,31,34,36-37H,4-5,7-8,11-12,15-21H2,1-3H3,(H,32,39)(H,33,38)/t25-,26-,27-,28+,31?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine cathepsin D |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012253
(CHEMBL56982 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES CCCCCCOC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)CC(C)C Show InChI InChI=1S/C36H60N4O9S/c1-4-5-6-13-20-49-36(45)32(35(44)37-29(24-27-14-9-7-10-15-27)33(42)31(41)23-26(2)3)38-34(43)30(25-28-16-11-8-12-17-28)39-50(46,47)40-18-21-48-22-19-40/h8,11-12,16-17,26-27,29-33,39,41-42H,4-7,9-10,13-15,18-25H2,1-3H3,(H,37,44)(H,38,43)/t29-,30-,31+,32?,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50005437

(CHEMBL8477 | N-[Allylsulfanyl-(1-cyclohexylmethyl-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)SCC=C Show InChI InChI=1S/C32H52N4O7S2/c1-4-19-44-32(31(40)33-26(21-24-11-7-5-8-12-24)29(38)28(37)20-23(2)3)34-30(39)27(22-25-13-9-6-10-14-25)35-45(41,42)36-15-17-43-18-16-36/h4,6,9-10,13-14,23-24,26-29,32,35,37-38H,1,5,7-8,11-12,15-22H2,2-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine cathepsin D |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50005443

(CHEMBL441325 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)OCC(F)(F)F Show InChI InChI=1S/C31H49F3N4O8S/c1-21(2)17-26(39)27(40)24(18-22-9-5-3-6-10-22)35-29(42)30(46-20-31(32,33)34)36-28(41)25(19-23-11-7-4-8-12-23)37-47(43,44)38-13-15-45-16-14-38/h4,7-8,11-12,21-22,24-27,30,37,39-40H,3,5-6,9-10,13-20H2,1-2H3,(H,35,42)(H,36,41)/t24-,25-,26-,27+,30?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine cathepsin D |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50005431

(CHEMBL8665 | N-[(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES CCCOC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C32H54N4O8S/c1-4-17-44-32(31(40)33-26(21-24-11-7-5-8-12-24)29(38)28(37)20-23(2)3)34-30(39)27(22-25-13-9-6-10-14-25)35-45(41,42)36-15-18-43-19-16-36/h6,9-10,13-14,23-24,26-29,32,35,37-38H,4-5,7-8,11-12,15-22H2,1-3H3,(H,33,40)(H,34,39)/t26-,27-,28-,29+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine cathepsin D |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50282993

((2S,4R)-4-Cyclohexyl-2-hydroxy-3-((R)-3-methylsulf...)Show SMILES CSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C(=O)OC(C)C Show InChI InChI=1S/C31H48N4O7S/c1-21(2)42-30(39)27(36)24(18-22-10-6-4-7-11-22)32-29(38)26(20-43-3)33-28(37)25(19-23-12-8-5-9-13-23)34-31(40)35-14-16-41-17-15-35/h5,8-9,12-13,21-22,24-27,36H,4,6-7,10-11,14-20H2,1-3H3,(H,32,38)(H,33,37)(H,34,40)/t24-,25-,26-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration on Bovine Cathepsin D |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005427
(4-[2-Ethoxy-2-(3-naphthalen-1-yl-2-naphthalen-1-yl...)Show SMILES CCOC(NC(=O)C(Cc1cccc2ccccc12)Cc1cccc2ccccc12)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)NC[C@@H](C)CC Show InChI InChI=1S/C41H53N3O5/c1-6-28(5)26-42-38(46)25-37(45)36(22-27(3)4)43-40(48)41(49-7-2)44-39(47)33(23-31-18-12-16-29-14-8-10-20-34(29)31)24-32-19-13-17-30-15-9-11-21-35(30)32/h8-21,27-28,33,36-37,41,45H,6-7,22-26H2,1-5H3,(H,42,46)(H,43,48)(H,44,47)/t28-,36+,37+,41?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50012250

(CHEMBL58947 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COC(=O)CC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)CC(C)C Show InChI InChI=1S/C32H52N4O9S/c1-22(2)18-28(37)30(39)25(19-23-10-6-4-7-11-23)33-31(40)26(21-29(38)44-3)34-32(41)27(20-24-12-8-5-9-13-24)35-46(42,43)36-14-16-45-17-15-36/h5,8-9,12-13,22-23,25-28,30,35,37,39H,4,6-7,10-11,14-21H2,1-3H3,(H,33,40)(H,34,41)/t25-,26?,27-,28+,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Bovine Cathepsin D. |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012254

(CHEMBL60535 | N-(1-Cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES CC(C)C[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(O)=O Show InChI InChI=1S/C30H48N4O9S/c1-20(2)17-25(35)27(36)23(18-21-9-5-3-6-10-21)31-29(38)26(30(39)40)32-28(37)24(19-22-11-7-4-8-12-22)33-44(41,42)34-13-15-43-16-14-34/h4,7-8,11-12,20-21,23-27,33,35-36H,3,5-6,9-10,13-19H2,1-2H3,(H,31,38)(H,32,37)(H,39,40)/t23-,24-,25+,26?,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of monkey plasma renin |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50005439

((1-{[(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...)Show SMILES CCSC(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C32H53N3O6S/c1-7-42-30(29(39)33-24(19-22-14-10-8-11-15-22)27(37)26(36)18-21(2)3)35-28(38)25(20-23-16-12-9-13-17-23)34-31(40)41-32(4,5)6/h9,12-13,16-17,21-22,24-27,30,36-37H,7-8,10-11,14-15,18-20H2,1-6H3,(H,33,39)(H,34,40)(H,35,38)/t24-,25-,26-,27+,30?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine cathepsin D |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50012247

(4-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-hexyl...)Show SMILES COC(=O)CCC(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)CC(C)C Show InChI InChI=1S/C33H54N4O9S/c1-23(2)20-29(38)31(40)27(21-24-10-6-4-7-11-24)35-32(41)26(14-15-30(39)45-3)34-33(42)28(22-25-12-8-5-9-13-25)36-47(43,44)37-16-18-46-19-17-37/h5,8-9,12-13,23-24,26-29,31,36,38,40H,4,6-7,10-11,14-22H2,1-3H3,(H,34,42)(H,35,41)/t26?,27-,28-,29+,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Bovine Cathepsin D. |
J Med Chem 34: 1935-43 (1991)
BindingDB Entry DOI: 10.7270/Q2NG4R73 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50282396
((S)-1-(4-Dimethylamino-3-methyl-benzyl)-5-diphenyl...)Show SMILES CN(C)c1ccc(Cn2cnc3CN([C@@H](Cc23)C(O)=O)C(=O)C(c2ccccc2)c2ccccc2)cc1C Show InChI InChI=1S/C31H32N4O3/c1-21-16-22(14-15-26(21)33(2)3)18-34-20-32-25-19-35(28(31(37)38)17-27(25)34)30(36)29(23-10-6-4-7-11-23)24-12-8-5-9-13-24/h4-16,20,28-29H,17-19H2,1-3H3,(H,37,38)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50005425

((1-{[(Allyl-methyl-amino)-(1-cyclohexylmethyl-2,3-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)N(C)CC=C Show InChI InChI=1S/C34H56N4O6/c1-8-19-38(7)30(32(42)35-26(21-24-15-11-9-12-16-24)29(40)28(39)20-23(2)3)37-31(41)27(22-25-17-13-10-14-18-25)36-33(43)44-34(4,5)6/h8,10,13-14,17-18,23-24,26-30,39-40H,1,9,11-12,15-16,19-22H2,2-7H3,(H,35,42)(H,36,43)(H,37,41)/t26-,27-,28-,29+,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey renin in vitro. |
J Med Chem 35: 1032-42 (1992)
BindingDB Entry DOI: 10.7270/Q2NG4PKR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data