 Found 1738 hits with Last Name = 'ho' and Initial = 'ml'
Found 1738 hits with Last Name = 'ho' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597587

(CHEMBL5174969)Show SMILES Fc1ccc2c(Cc3nnc(Cc4cccc(Cl)c4)n3CCCc3c[nH]cn3)c[nH]c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597579

(CHEMBL5197201)Show SMILES Clc1cccc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597581
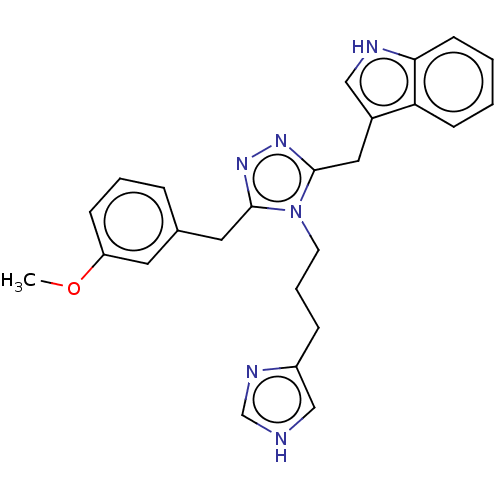
(CHEMBL5183772)Show SMILES COc1cccc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597590

(CHEMBL5185547)Show SMILES COc1cccc(Cc2nnc(Cc3c[nH]c4cc(F)ccc34)n2CCCc2c[nH]cn2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597580
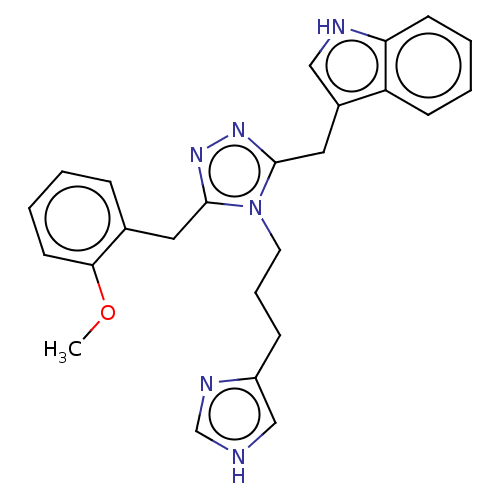
(CHEMBL5179456)Show SMILES COc1ccccc1Cc1nnc(Cc2c[nH]c3ccccc23)n1CCCc1c[nH]cn1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523770

(US11136312, Compound SK-I-124)Show SMILES Fc1ccc2c(Cc3nnc(Cc4c[nH]c5cc(F)ccc45)n3CCCc3c[nH]cn3)c[nH]c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523757

(US11136312, Compound MM-I-87)Show SMILES Fc1ccc2c(Cc3nnc(Cc4cccc(c4)C(F)(F)F)n3CCCc3c[nH]cn3)c[nH]c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597591

(CHEMBL5172814)Show SMILES CS(=O)(=O)c1cccc(Cc2nnc(Cc3c[nH]c4cc(F)ccc34)n2CCCc2c[nH]cn2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC6 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597576

(CHEMBL5180543)Show SMILES Clc1ccc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)cc1Cl | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523754

(US11136312, Compound MM-I-66)Show SMILES FC(F)(F)c1cccc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401001
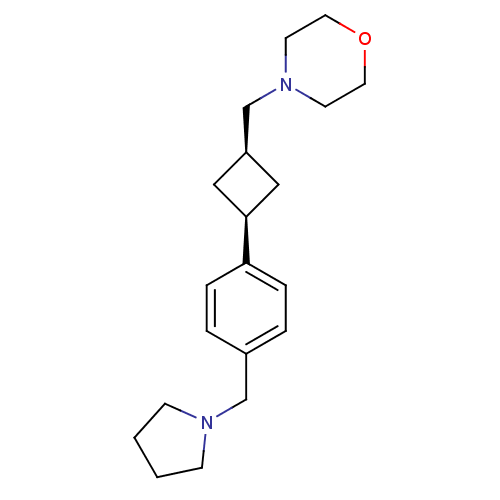
(CHEMBL2206288)Show SMILES C([C@H]1C[C@H](C1)c1ccc(CN2CCCC2)cc1)N1CCOCC1 |r,wU:3.5,1.0,(12.44,-17.63,;13.93,-17.23,;14.7,-15.89,;16.03,-16.66,;15.26,-18,;17.52,-16.26,;18.61,-17.35,;20.1,-16.95,;20.5,-15.47,;21.98,-15.07,;23.07,-16.16,;22.83,-17.68,;24.2,-18.38,;25.29,-17.29,;24.59,-15.92,;19.41,-14.38,;17.92,-14.78,;11.35,-16.54,;11.75,-15.05,;10.66,-13.96,;9.18,-14.36,;8.78,-15.85,;9.87,-16.93,)| Show InChI InChI=1S/C20H30N2O/c1-2-8-21(7-1)15-17-3-5-19(6-4-17)20-13-18(14-20)16-22-9-11-23-12-10-22/h3-6,18,20H,1-2,7-16H2/t18-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401001

(CHEMBL2206288)Show SMILES C([C@H]1C[C@H](C1)c1ccc(CN2CCCC2)cc1)N1CCOCC1 |r,wU:3.5,1.0,(12.44,-17.63,;13.93,-17.23,;14.7,-15.89,;16.03,-16.66,;15.26,-18,;17.52,-16.26,;18.61,-17.35,;20.1,-16.95,;20.5,-15.47,;21.98,-15.07,;23.07,-16.16,;22.83,-17.68,;24.2,-18.38,;25.29,-17.29,;24.59,-15.92,;19.41,-14.38,;17.92,-14.78,;11.35,-16.54,;11.75,-15.05,;10.66,-13.96,;9.18,-14.36,;8.78,-15.85,;9.87,-16.93,)| Show InChI InChI=1S/C20H30N2O/c1-2-8-21(7-1)15-17-3-5-19(6-4-17)20-13-18(14-20)16-22-9-11-23-12-10-22/h3-6,18,20H,1-2,7-16H2/t18-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597589

(CHEMBL5209180)Show SMILES COc1ccccc1Cc1nnc(Cc2c[nH]c3cc(F)ccc23)n1CCCc1c[nH]cn1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523767
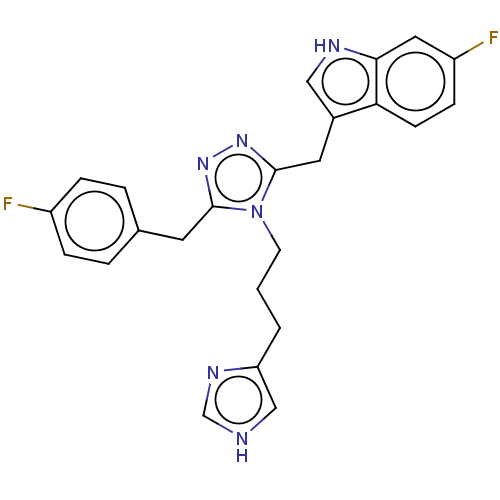
(US11136312, Compound SK-I-91)Show SMILES Fc1ccc(Cc2nnc(Cc3c[nH]c4cc(F)ccc34)n2CCCc2c[nH]cn2)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523756

(US11136312, Compound MM-I-83)Show SMILES Fc1ccc2c(Cc3nnc(Cc4cccc(Br)c4)n3CCCc3c[nH]cn3)c[nH]c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523755

(US11136312, Compound MM-I-72)Show SMILES CS(=O)(=O)c1cccc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597585

(CHEMBL5176083)Show SMILES Fc1ccc2c(Cc3nnc(Cc4ccc(Cl)c(Cl)c4)n3CCCc3c[nH]cn3)c[nH]c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401002
(CHEMBL2206292)Show SMILES CC(C)CNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:7.6,wD:9.9,(6.68,-19.62,;6.28,-18.13,;4.79,-17.73,;7.37,-17.04,;8.85,-17.44,;9.94,-16.35,;9.54,-14.87,;11.43,-16.75,;12.76,-15.98,;13.53,-17.32,;14.3,-18.65,;12.2,-18.09,;14.87,-16.55,;14.87,-15.01,;16.2,-14.24,;17.54,-15.01,;18.87,-14.24,;20.2,-15.01,;20.36,-16.54,;21.87,-16.86,;22.64,-15.52,;21.61,-14.38,;17.54,-16.55,;18.87,-17.32,;16.2,-17.32,)| Show InChI InChI=1S/C20H28F2N2O/c1-14(2)12-23-19(25)16-10-20(22,11-16)17-6-5-15(18(21)9-17)13-24-7-3-4-8-24/h5-6,9,14,16H,3-4,7-8,10-13H2,1-2H3,(H,23,25)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401002

(CHEMBL2206292)Show SMILES CC(C)CNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:7.6,wD:9.9,(6.68,-19.62,;6.28,-18.13,;4.79,-17.73,;7.37,-17.04,;8.85,-17.44,;9.94,-16.35,;9.54,-14.87,;11.43,-16.75,;12.76,-15.98,;13.53,-17.32,;14.3,-18.65,;12.2,-18.09,;14.87,-16.55,;14.87,-15.01,;16.2,-14.24,;17.54,-15.01,;18.87,-14.24,;20.2,-15.01,;20.36,-16.54,;21.87,-16.86,;22.64,-15.52,;21.61,-14.38,;17.54,-16.55,;18.87,-17.32,;16.2,-17.32,)| Show InChI InChI=1S/C20H28F2N2O/c1-14(2)12-23-19(25)16-10-20(22,11-16)17-6-5-15(18(21)9-17)13-24-7-3-4-8-24/h5-6,9,14,16H,3-4,7-8,10-13H2,1-2H3,(H,23,25)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597577
(CHEMBL5190056)Show SMILES Clc1cc(Cl)cc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC9 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401007
(CHEMBL209478)Show InChI InChI=1S/C18H24N2/c1-19(2)13-15-5-9-17(10-6-15)18-11-7-16(8-12-18)14-20(3)4/h5-12H,13-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401007

(CHEMBL209478)Show InChI InChI=1S/C18H24N2/c1-19(2)13-15-5-9-17(10-6-15)18-11-7-16(8-12-18)14-20(3)4/h5-12H,13-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401002

(CHEMBL2206292)Show SMILES CC(C)CNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:7.6,wD:9.9,(6.68,-19.62,;6.28,-18.13,;4.79,-17.73,;7.37,-17.04,;8.85,-17.44,;9.94,-16.35,;9.54,-14.87,;11.43,-16.75,;12.76,-15.98,;13.53,-17.32,;14.3,-18.65,;12.2,-18.09,;14.87,-16.55,;14.87,-15.01,;16.2,-14.24,;17.54,-15.01,;18.87,-14.24,;20.2,-15.01,;20.36,-16.54,;21.87,-16.86,;22.64,-15.52,;21.61,-14.38,;17.54,-16.55,;18.87,-17.32,;16.2,-17.32,)| Show InChI InChI=1S/C20H28F2N2O/c1-14(2)12-23-19(25)16-10-20(22,11-16)17-6-5-15(18(21)9-17)13-24-7-3-4-8-24/h5-6,9,14,16H,3-4,7-8,10-13H2,1-2H3,(H,23,25)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523760

(US11136312, Compound SK-I-22)Show SMILES Clc1ccc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401002

(CHEMBL2206292)Show SMILES CC(C)CNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:7.6,wD:9.9,(6.68,-19.62,;6.28,-18.13,;4.79,-17.73,;7.37,-17.04,;8.85,-17.44,;9.94,-16.35,;9.54,-14.87,;11.43,-16.75,;12.76,-15.98,;13.53,-17.32,;14.3,-18.65,;12.2,-18.09,;14.87,-16.55,;14.87,-15.01,;16.2,-14.24,;17.54,-15.01,;18.87,-14.24,;20.2,-15.01,;20.36,-16.54,;21.87,-16.86,;22.64,-15.52,;21.61,-14.38,;17.54,-16.55,;18.87,-17.32,;16.2,-17.32,)| Show InChI InChI=1S/C20H28F2N2O/c1-14(2)12-23-19(25)16-10-20(22,11-16)17-6-5-15(18(21)9-17)13-24-7-3-4-8-24/h5-6,9,14,16H,3-4,7-8,10-13H2,1-2H3,(H,23,25)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401004

(CHEMBL2206291)Show SMILES CCN(C)C(=O)[C@H]1C[C@H](C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:6.5,8.10,(5.81,-12.97,;6.58,-14.3,;8.12,-14.3,;8.89,-12.97,;8.89,-15.64,;8.12,-16.97,;10.43,-15.64,;11.51,-14.55,;12.6,-15.64,;11.51,-16.72,;14.14,-15.64,;14.91,-14.3,;16.45,-14.3,;17.22,-15.64,;18.76,-15.64,;19.53,-14.3,;21.06,-14.14,;21.39,-12.63,;20.05,-11.86,;18.91,-12.89,;16.45,-16.97,;17.22,-18.3,;14.91,-16.97,)| Show InChI InChI=1S/C19H27FN2O/c1-3-21(2)19(23)17-10-16(11-17)14-6-7-15(18(20)12-14)13-22-8-4-5-9-22/h6-7,12,16-17H,3-5,8-11,13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401004

(CHEMBL2206291)Show SMILES CCN(C)C(=O)[C@H]1C[C@H](C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:6.5,8.10,(5.81,-12.97,;6.58,-14.3,;8.12,-14.3,;8.89,-12.97,;8.89,-15.64,;8.12,-16.97,;10.43,-15.64,;11.51,-14.55,;12.6,-15.64,;11.51,-16.72,;14.14,-15.64,;14.91,-14.3,;16.45,-14.3,;17.22,-15.64,;18.76,-15.64,;19.53,-14.3,;21.06,-14.14,;21.39,-12.63,;20.05,-11.86,;18.91,-12.89,;16.45,-16.97,;17.22,-18.3,;14.91,-16.97,)| Show InChI InChI=1S/C19H27FN2O/c1-3-21(2)19(23)17-10-16(11-17)14-6-7-15(18(20)12-14)13-22-8-4-5-9-22/h6-7,12,16-17H,3-5,8-11,13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401003
(CHEMBL2151197)Show SMILES CCNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:5.4,wD:7.7,(15.4,-34.66,;16.73,-35.43,;18.07,-34.66,;19.4,-35.43,;19.4,-36.97,;20.73,-34.66,;21.13,-33.17,;22.62,-33.57,;23.84,-34.51,;22.22,-35.06,;23.95,-32.81,;25.29,-33.58,;26.62,-32.81,;26.62,-31.26,;27.95,-30.48,;29.28,-31.25,;29.46,-32.78,;30.97,-33.09,;31.73,-31.76,;30.7,-30.62,;25.28,-30.5,;25.28,-28.96,;23.95,-31.27,)| Show InChI InChI=1S/C18H24F2N2O/c1-2-21-17(23)14-10-18(20,11-14)15-6-5-13(16(19)9-15)12-22-7-3-4-8-22/h5-6,9,14H,2-4,7-8,10-12H2,1H3,(H,21,23)/t14-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597586
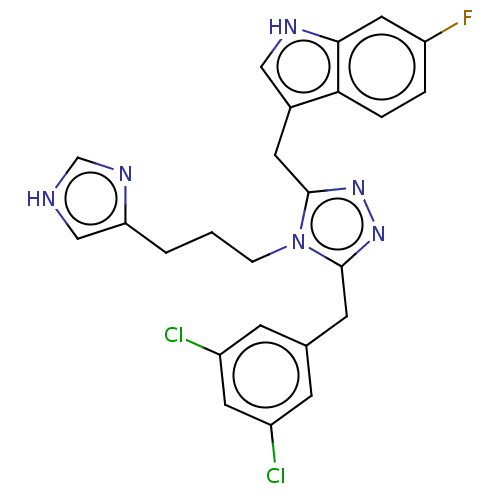
(CHEMBL5173784)Show SMILES Fc1ccc2c(Cc3nnc(Cc4cc(Cl)cc(Cl)c4)n3CCCc3c[nH]cn3)c[nH]c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523759
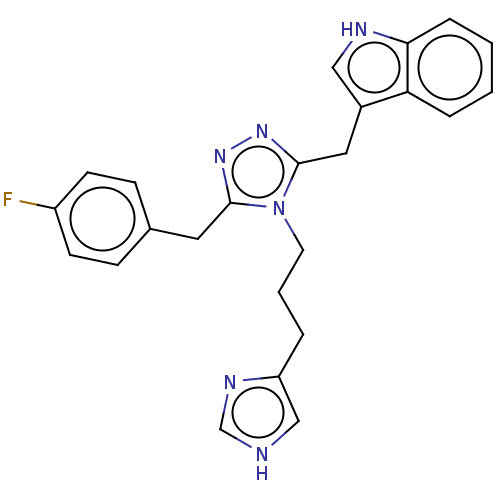
(US11136312, Compound SK-I-16)Show SMILES Fc1ccc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523762

(US11136312, Compound SK-I-25)Show SMILES C(Cc1c[nH]cn1)Cn1c(Cc2c[nH]c3ccccc23)nnc1Cc1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401003

(CHEMBL2151197)Show SMILES CCNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:5.4,wD:7.7,(15.4,-34.66,;16.73,-35.43,;18.07,-34.66,;19.4,-35.43,;19.4,-36.97,;20.73,-34.66,;21.13,-33.17,;22.62,-33.57,;23.84,-34.51,;22.22,-35.06,;23.95,-32.81,;25.29,-33.58,;26.62,-32.81,;26.62,-31.26,;27.95,-30.48,;29.28,-31.25,;29.46,-32.78,;30.97,-33.09,;31.73,-31.76,;30.7,-30.62,;25.28,-30.5,;25.28,-28.96,;23.95,-31.27,)| Show InChI InChI=1S/C18H24F2N2O/c1-2-21-17(23)14-10-18(20,11-14)15-6-5-13(16(19)9-15)12-22-7-3-4-8-22/h5-6,9,14H,2-4,7-8,10-12H2,1H3,(H,21,23)/t14-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523766
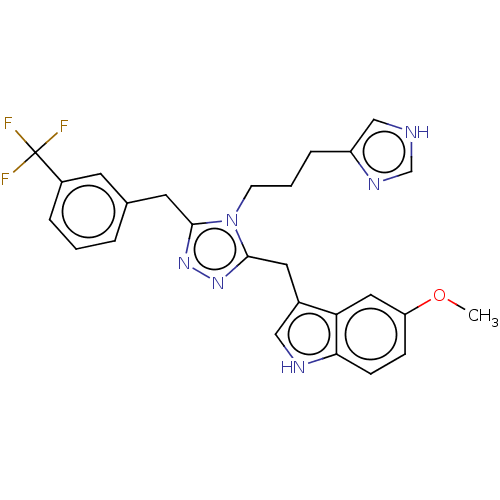
(US11136312, Compound SK-I-57)Show SMILES COc1ccc2[nH]cc(Cc3nnc(Cc4cccc(c4)C(F)(F)F)n3CCCc3c[nH]cn3)c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC2 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401001

(CHEMBL2206288)Show SMILES C([C@H]1C[C@H](C1)c1ccc(CN2CCCC2)cc1)N1CCOCC1 |r,wU:3.5,1.0,(12.44,-17.63,;13.93,-17.23,;14.7,-15.89,;16.03,-16.66,;15.26,-18,;17.52,-16.26,;18.61,-17.35,;20.1,-16.95,;20.5,-15.47,;21.98,-15.07,;23.07,-16.16,;22.83,-17.68,;24.2,-18.38,;25.29,-17.29,;24.59,-15.92,;19.41,-14.38,;17.92,-14.78,;11.35,-16.54,;11.75,-15.05,;10.66,-13.96,;9.18,-14.36,;8.78,-15.85,;9.87,-16.93,)| Show InChI InChI=1S/C20H30N2O/c1-2-8-21(7-1)15-17-3-5-19(6-4-17)20-13-18(14-20)16-22-9-11-23-12-10-22/h3-6,18,20H,1-2,7-16H2/t18-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523761

(US11136312, Compound SK-I-23)Show SMILES COc1ccc(Cc2nnc(Cc3c[nH]c4ccccc34)n2CCCc2c[nH]cn2)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401001

(CHEMBL2206288)Show SMILES C([C@H]1C[C@H](C1)c1ccc(CN2CCCC2)cc1)N1CCOCC1 |r,wU:3.5,1.0,(12.44,-17.63,;13.93,-17.23,;14.7,-15.89,;16.03,-16.66,;15.26,-18,;17.52,-16.26,;18.61,-17.35,;20.1,-16.95,;20.5,-15.47,;21.98,-15.07,;23.07,-16.16,;22.83,-17.68,;24.2,-18.38,;25.29,-17.29,;24.59,-15.92,;19.41,-14.38,;17.92,-14.78,;11.35,-16.54,;11.75,-15.05,;10.66,-13.96,;9.18,-14.36,;8.78,-15.85,;9.87,-16.93,)| Show InChI InChI=1S/C20H30N2O/c1-2-8-21(7-1)15-17-3-5-19(6-4-17)20-13-18(14-20)16-22-9-11-23-12-10-22/h3-6,18,20H,1-2,7-16H2/t18-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401003

(CHEMBL2151197)Show SMILES CCNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:5.4,wD:7.7,(15.4,-34.66,;16.73,-35.43,;18.07,-34.66,;19.4,-35.43,;19.4,-36.97,;20.73,-34.66,;21.13,-33.17,;22.62,-33.57,;23.84,-34.51,;22.22,-35.06,;23.95,-32.81,;25.29,-33.58,;26.62,-32.81,;26.62,-31.26,;27.95,-30.48,;29.28,-31.25,;29.46,-32.78,;30.97,-33.09,;31.73,-31.76,;30.7,-30.62,;25.28,-30.5,;25.28,-28.96,;23.95,-31.27,)| Show InChI InChI=1S/C18H24F2N2O/c1-2-21-17(23)14-10-18(20,11-14)15-6-5-13(16(19)9-15)12-22-7-3-4-8-22/h5-6,9,14H,2-4,7-8,10-12H2,1H3,(H,21,23)/t14-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401003

(CHEMBL2151197)Show SMILES CCNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:5.4,wD:7.7,(15.4,-34.66,;16.73,-35.43,;18.07,-34.66,;19.4,-35.43,;19.4,-36.97,;20.73,-34.66,;21.13,-33.17,;22.62,-33.57,;23.84,-34.51,;22.22,-35.06,;23.95,-32.81,;25.29,-33.58,;26.62,-32.81,;26.62,-31.26,;27.95,-30.48,;29.28,-31.25,;29.46,-32.78,;30.97,-33.09,;31.73,-31.76,;30.7,-30.62,;25.28,-30.5,;25.28,-28.96,;23.95,-31.27,)| Show InChI InChI=1S/C18H24F2N2O/c1-2-21-17(23)14-10-18(20,11-14)15-6-5-13(16(19)9-15)12-22-7-3-4-8-22/h5-6,9,14H,2-4,7-8,10-12H2,1H3,(H,21,23)/t14-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC3 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC7 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50353233
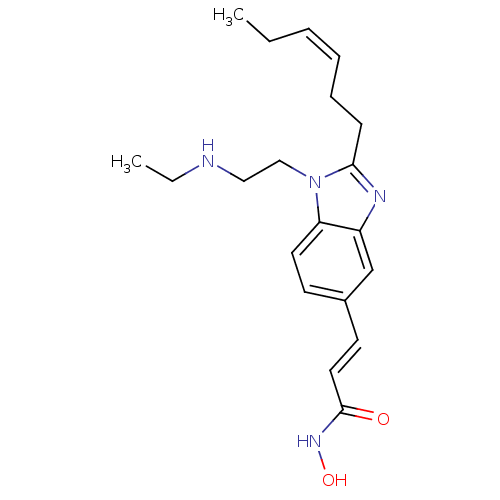
(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC1 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50597578

(CHEMBL5205998)Show SMILES Clc1ccccc1Cc1nnc(Cc2c[nH]c3ccccc23)n1CCCc1c[nH]cn1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523764
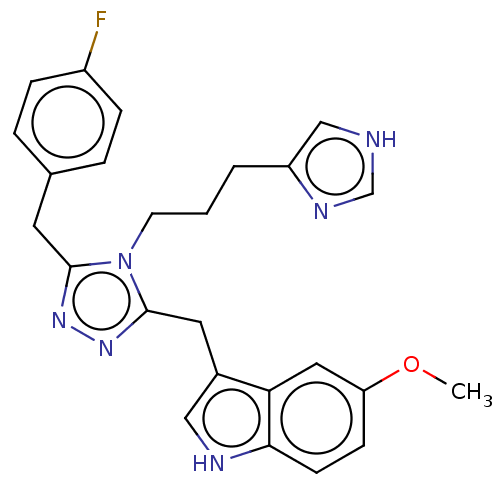
(US11136312, Compound SK-I-55)Show SMILES COc1ccc2[nH]cc(Cc3nnc(Cc4ccc(F)cc4)n3CCCc3c[nH]cn3)c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM523758

(US11136312, Compound MM-I-89)Show SMILES Fc1ccc2c(Cc3nnc(Cc4ccccc4)n3CCCc3c[nH]cn3)c[nH]c2c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00044f
BindingDB Entry DOI: 10.7270/Q21C21X0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data