

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50236985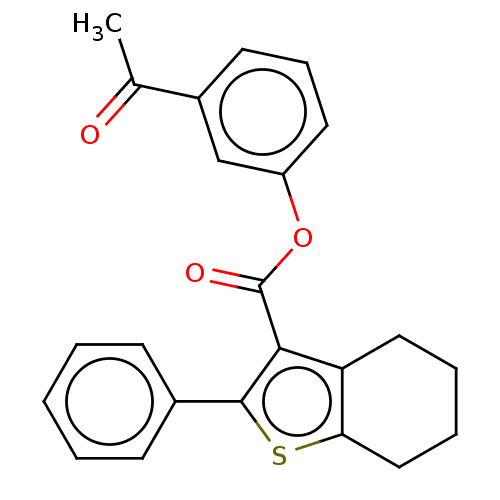 (CHEMBL4098600) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50236980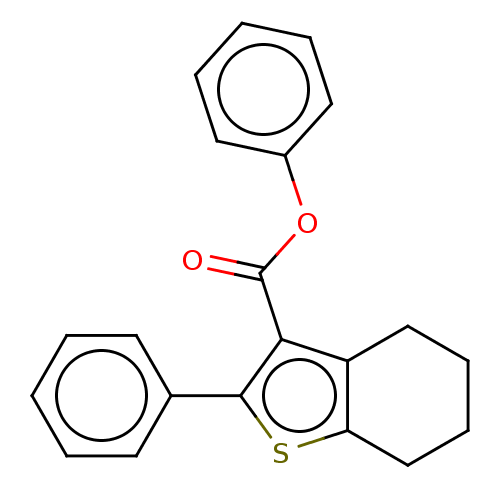 (CHEMBL4097499) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50236981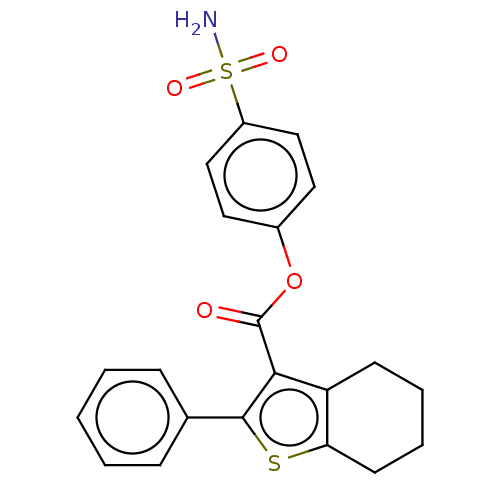 (CHEMBL4086580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50236984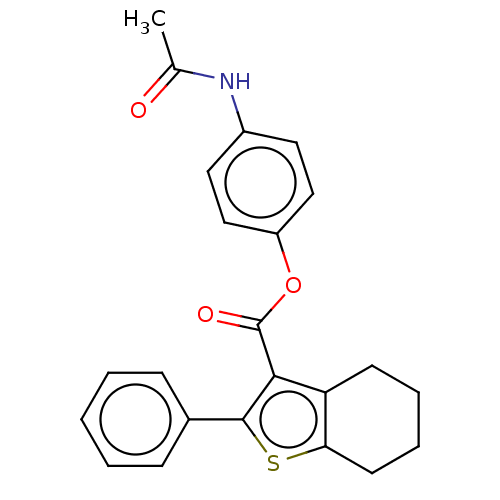 (CHEMBL4069250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by ADHP pro... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50236985 (CHEMBL4098600) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by ADHP pro... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50236981 (CHEMBL4086580) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by ADHP pro... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50236980 (CHEMBL4097499) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by ADHP pro... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50236984 (CHEMBL4069250) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by ADHP pro... | Bioorg Med Chem Lett 27: 1721-1726 (2017) Article DOI: 10.1016/j.bmcl.2017.02.076 BindingDB Entry DOI: 10.7270/Q2348NNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||