 Found 36 hits with Last Name = 'ishikawa' and Initial = 'a'
Found 36 hits with Last Name = 'ishikawa' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615884

(CHEMBL5279603)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccc(N)cc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615889
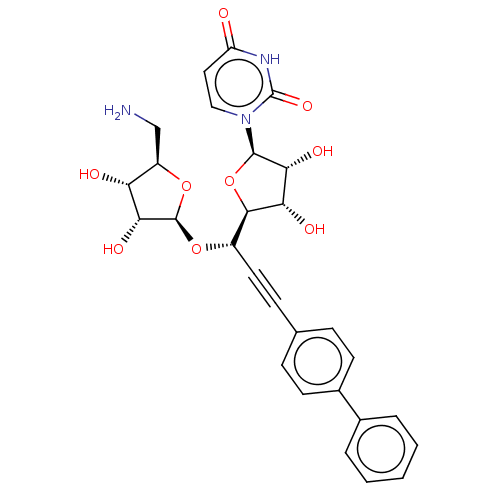
(CHEMBL5289885)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccc(cc2)-c2ccccc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615888
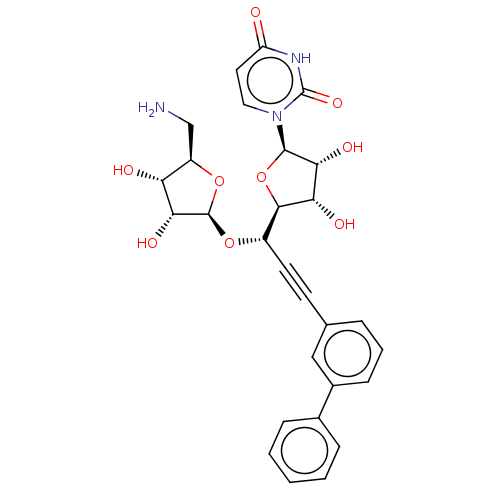
(CHEMBL5277496)Show SMILES [H][C@@]1(O[C@@H](C#Cc2cccc(c2)-c2ccccc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615893

(CHEMBL5287014)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccc(cc2)C(=O)Nc2ccccc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615880

(CHEMBL5268197)Show SMILES [H][C@@]1(O[C@@H](C#Cc2cccc(NS(=O)(=O)c3ccccc3)c2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615894
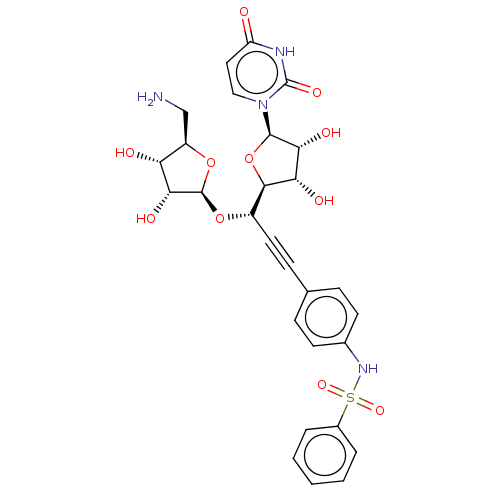
(CHEMBL5291217)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccc(NS(=O)(=O)c3ccccc3)cc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615892
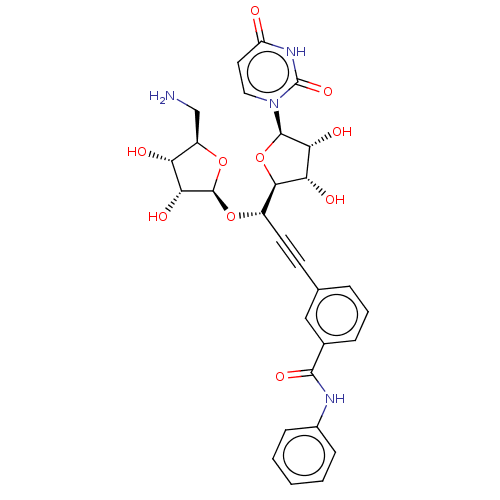
(CHEMBL5269635)Show SMILES [H][C@@]1(O[C@@H](C#Cc2cccc(c2)C(=O)Nc2ccccc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615883
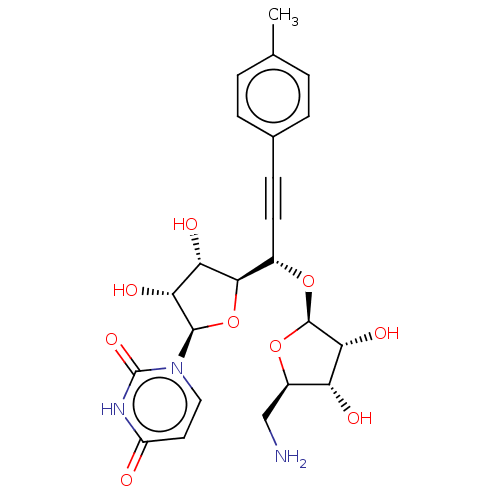
(CHEMBL5288794)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccc(C)cc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615879
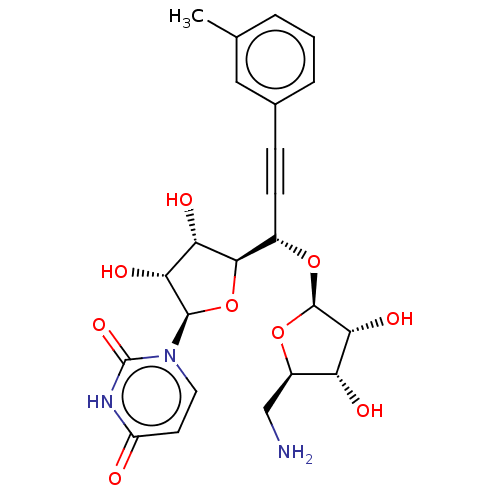
(CHEMBL5283961)Show SMILES [H][C@@]1(O[C@@H](C#Cc2cccc(C)c2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615891

(CHEMBL5278736)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccc(NC(=O)c3ccccc3)cc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615886

(CHEMBL5266213)Show SMILES [H][C@@]1(O[C@@H](C#Cc2cccc(c2)C(O)=O)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615881

(CHEMBL5290020)Show SMILES [H][C@@]1(O[C@@H](C#Cc2cccc(N)c2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615878

(CHEMBL5274543)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccccc2C)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50600491

(CHEMBL5209278)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)[C@@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)C#Cc1ccccc1 |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615882

(CHEMBL5274523)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccccc2N)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290537
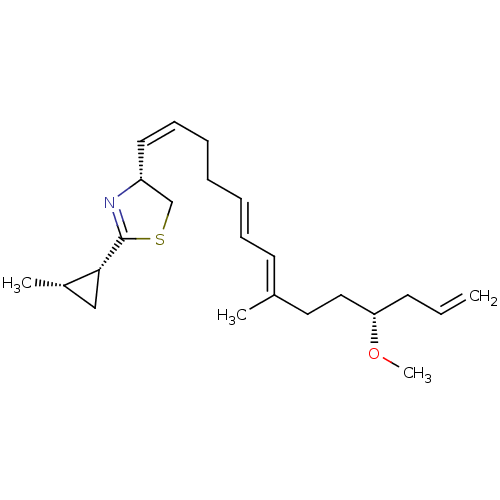
((R)-4-((1Z,5E,7E)-(R)-11-Methoxy-8-methyl-tetradec...)Show SMILES CO[C@H](CC\C(C)=C\C=C\CC\C=C/[C@@H]1CSC(=N1)[C@@H]1C[C@@H]1C)CC=C |r,c:17| Show InChI InChI=1S/C23H35NOS/c1-5-11-21(25-4)15-14-18(2)12-9-7-6-8-10-13-20-17-26-23(24-20)22-16-19(22)3/h5,7,9-10,12-13,19-22H,1,6,8,11,14-17H2,2-4H3/b9-7+,13-10-,18-12+/t19-,20+,21-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its anti tubulin activity |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290537

((R)-4-((1Z,5E,7E)-(R)-11-Methoxy-8-methyl-tetradec...)Show SMILES CO[C@H](CC\C(C)=C\C=C\CC\C=C/[C@@H]1CSC(=N1)[C@@H]1C[C@@H]1C)CC=C |r,c:17| Show InChI InChI=1S/C23H35NOS/c1-5-11-21(25-4)15-14-18(2)12-9-7-6-8-10-13-20-17-26-23(24-20)22-16-19(22)3/h5,7,9-10,12-13,19-22H,1,6,8,11,14-17H2,2-4H3/b9-7+,13-10-,18-12+/t19-,20+,21-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against tubulin polymerization |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50005480

((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro tubulin polymerization-inhibitory activity. |
Bioorg Med Chem Lett 8: 1997-2000 (1998)
BindingDB Entry DOI: 10.7270/Q2VH5R10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta chain
(Sus scrofa) | BDBM50216141
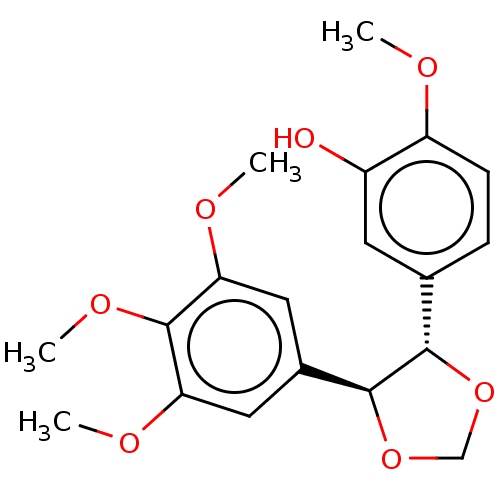
(CHEMBL61572)Show SMILES COc1ccc(cc1O)[C@@H]1OCO[C@H]1c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H22O7/c1-21-14-6-5-11(7-13(14)20)17-18(26-10-25-17)12-8-15(22-2)19(24-4)16(9-12)23-3/h5-9,17-18,20H,10H2,1-4H3/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro tubulin polymerization-inhibitory activity; 4-6 |
Bioorg Med Chem Lett 8: 1997-2000 (1998)
BindingDB Entry DOI: 10.7270/Q2VH5R10 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615887

(CHEMBL5265879)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccc(cc2)C(O)=O)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615885

(CHEMBL5286726)Show SMILES [H][C@@]1(O[C@@H](C#Cc2ccccc2C(O)=O)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615890

(CHEMBL5277965)Show SMILES [H][C@@]1(O[C@@H](C#Cc2cccc(NC(=O)c3ccccc3)c2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50014846
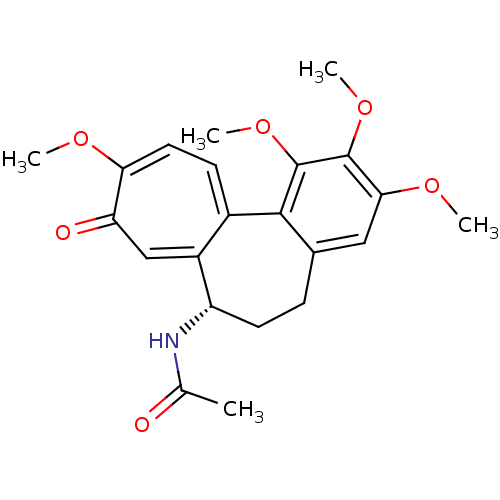
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro tubulin polymerization-inhibitory activity. |
Bioorg Med Chem Lett 8: 1997-2000 (1998)
BindingDB Entry DOI: 10.7270/Q2VH5R10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50615877

(CHEMBL5273400)Show SMILES [H][C@@]1(O[C@@H](CCc2ccccc2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50216137
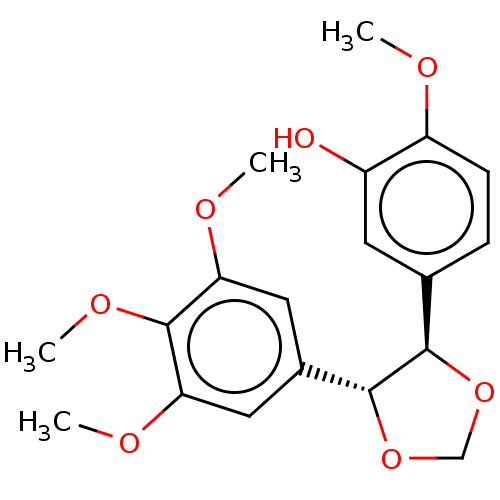
(CHEMBL303103)Show SMILES COc1ccc(cc1O)[C@H]1OCO[C@@H]1c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H22O7/c1-21-14-6-5-11(7-13(14)20)17-18(26-10-25-17)12-8-15(22-2)19(24-4)16(9-12)23-3/h5-9,17-18,20H,10H2,1-4H3/t17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
In vitro tubulin polymerization-inhibitory activity. |
Bioorg Med Chem Lett 8: 1997-2000 (1998)
BindingDB Entry DOI: 10.7270/Q2VH5R10 |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290542
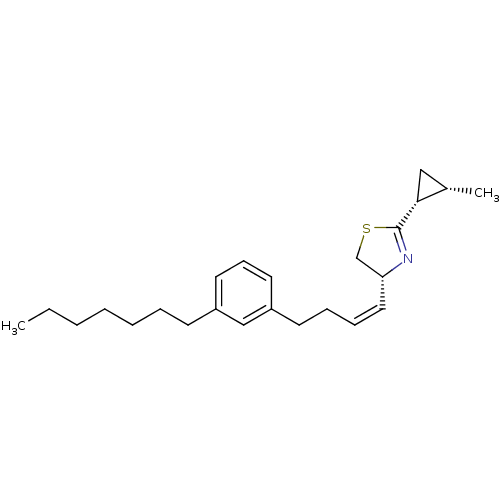
((R)-4-[(Z)-4-(3-Heptyl-phenyl)-but-1-enyl]-2-((1R,...)Show SMILES CCCCCCCc1cccc(CC\C=C/[C@@H]2CSC(=N2)[C@@H]2C[C@@H]2C)c1 |c:19| Show InChI InChI=1S/C24H35NS/c1-3-4-5-6-7-11-20-13-10-14-21(17-20)12-8-9-15-22-18-26-24(25-22)23-16-19(23)2/h9-10,13-15,17,19,22-23H,3-8,11-12,16,18H2,1-2H3/b15-9-/t19-,22+,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against tubulin polymerization |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290541

((R)-4-[(Z)-4-(4-Hexyl-phenyl)-but-1-enyl]-2-((1R,2...)Show SMILES CCCCCCc1ccc(CC\C=C/[C@@H]2CSC(=N2)[C@@H]2C[C@@H]2C)cc1 |c:17| Show InChI InChI=1S/C23H33NS/c1-3-4-5-6-9-19-12-14-20(15-13-19)10-7-8-11-21-17-25-23(24-21)22-16-18(22)2/h8,11-15,18,21-22H,3-7,9-10,16-17H2,1-2H3/b11-8-/t18-,21+,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against tubulin polymerization |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290539
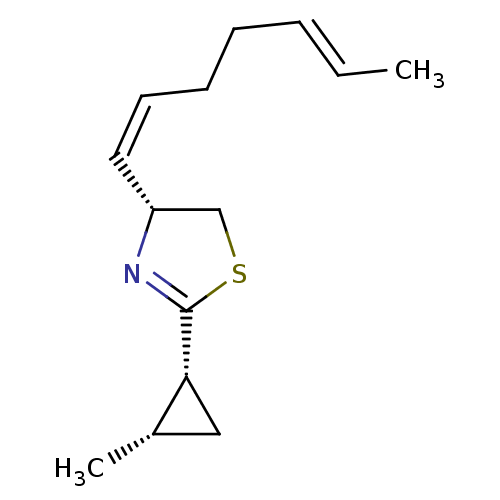
((R)-4-((1Z,5E)-Hepta-1,5-dienyl)-2-((1R,2S)-2-meth...)Show SMILES C\C=C\CC\C=C/[C@@H]1CSC(=N1)[C@@H]1C[C@@H]1C |c:10| Show InChI InChI=1S/C14H21NS/c1-3-4-5-6-7-8-12-10-16-14(15-12)13-9-11(13)2/h3-4,7-8,11-13H,5-6,9-10H2,1-2H3/b4-3+,8-7-/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against tubulin polymerization |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290538
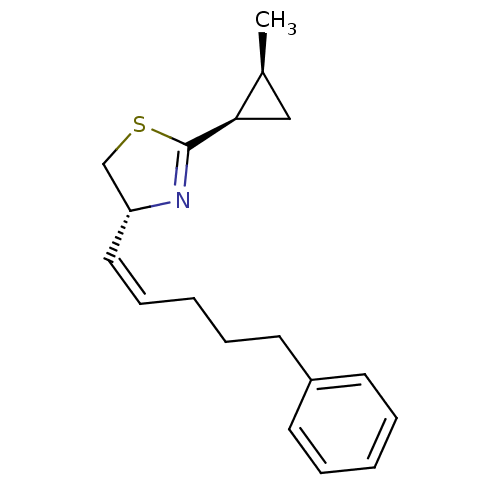
((R)-2-((1R,2S)-2-Methyl-cyclopropyl)-4-((Z)-5-phen...)Show SMILES C[C@H]1C[C@H]1C1=N[C@@H](CS1)\C=C/CCCc1ccccc1 |t:5| Show InChI InChI=1S/C18H23NS/c1-14-12-17(14)18-19-16(13-20-18)11-7-3-6-10-15-8-4-2-5-9-15/h2,4-5,7-9,11,14,16-17H,3,6,10,12-13H2,1H3/b11-7-/t14-,16+,17+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against tubulin polymerization |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290536
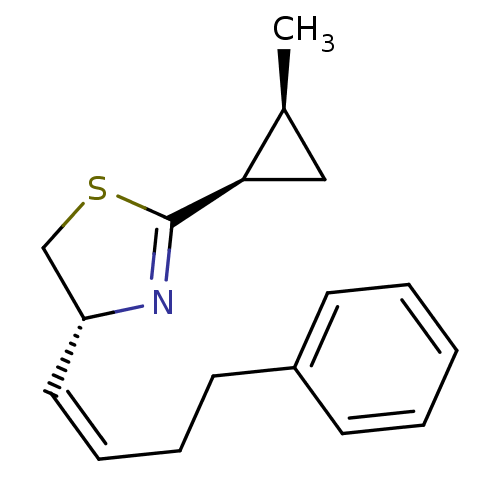
((R)-2-((1R,2S)-2-Methyl-cyclopropyl)-4-((Z)-4-phen...)Show SMILES C[C@H]1C[C@H]1C1=N[C@@H](CS1)\C=C/CCc1ccccc1 |t:5| Show InChI InChI=1S/C17H21NS/c1-13-11-16(13)17-18-15(12-19-17)10-6-5-9-14-7-3-2-4-8-14/h2-4,6-8,10,13,15-16H,5,9,11-12H2,1H3/b10-6-/t13-,15+,16+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against tubulin polymerization |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290534
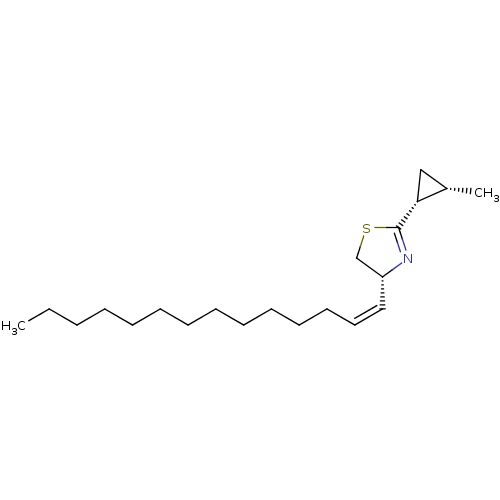
((R)-2-((1R,2S)-2-Methyl-cyclopropyl)-4-((Z)-tetrad...)Show SMILES CCCCCCCCCCCC\C=C/[C@@H]1CSC(=N1)[C@@H]1C[C@@H]1C |c:17| Show InChI InChI=1S/C21H37NS/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-17-23-21(22-19)20-16-18(20)2/h14-15,18-20H,3-13,16-17H2,1-2H3/b15-14-/t18-,19+,20+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against tubulin polymerization |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50290535
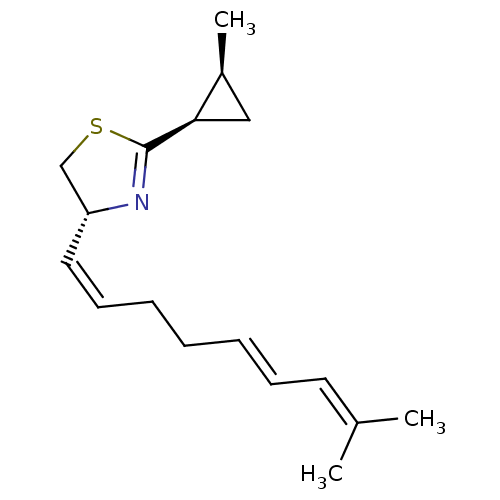
((R)-2-((1R,2S)-2-Methyl-cyclopropyl)-4-((1Z,5E)-8-...)Show SMILES [#6]-[#6@H]-1-[#6]-[#6@H]-1-[#6]-1=[#7]-[#6@@H](-[#6]-[#16]-1)\[#6]=[#6]/[#6]-[#6]\[#6]=[#6]\[#6]=[#6](\[#6])-[#6] |t:5| Show InChI InChI=1S/C17H25NS/c1-13(2)9-7-5-4-6-8-10-15-12-19-17(18-15)16-11-14(16)3/h5,7-10,14-16H,4,6,11-12H2,1-3H3/b7-5+,10-8-/t14-,15+,16+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against tubulin polymerization |
Bioorg Med Chem Lett 7: 2657-2660 (1997)
Article DOI: 10.1016/S0960-894X(97)10055-5
BindingDB Entry DOI: 10.7270/Q27081XB |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50615895
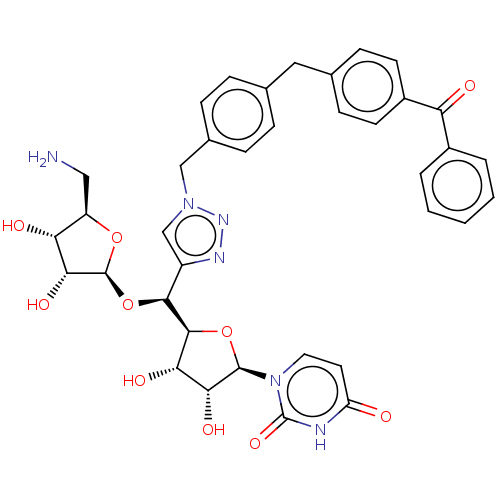
(CHEMBL5288357)Show SMILES [H][C@@]1(O[C@@H](c2cn(Cc3ccc(Cc4ccc(cc4)C(=O)c4ccccc4)cc3)nn2)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50526649
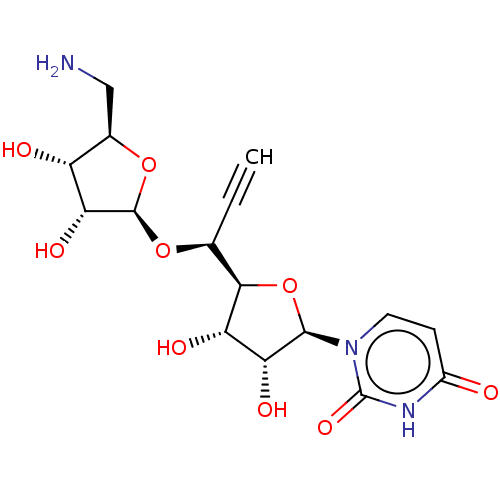
(CHEMBL4556639)Show SMILES [H][C@@]1(O[C@@H](C#C)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H21N3O9/c1-2-6(26-15-12(24)9(21)7(5-17)27-15)13-10(22)11(23)14(28-13)19-4-3-8(20)18-16(19)25/h1,3-4,6-7,9-15,21-24H,5,17H2,(H,18,20,25)/t6-,7+,9+,10-,11+,12+,13+,14+,15+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50526649

(CHEMBL4556639)Show SMILES [H][C@@]1(O[C@@H](C#C)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H21N3O9/c1-2-6(26-15-12(24)9(21)7(5-17)27-15)13-10(22)11(23)14(28-13)19-4-3-8(20)18-16(19)25/h1,3-4,6-7,9-15,21-24H,5,17H2,(H,18,20,25)/t6-,7+,9+,10-,11+,12+,13+,14+,15+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50526649

(CHEMBL4556639)Show SMILES [H][C@@]1(O[C@@H](C#C)[C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H21N3O9/c1-2-6(26-15-12(24)9(21)7(5-17)27-15)13-10(22)11(23)14(28-13)19-4-3-8(20)18-16(19)25/h1,3-4,6-7,9-15,21-24H,5,17H2,(H,18,20,25)/t6-,7+,9+,10-,11+,12+,13+,14+,15+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data