

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50085072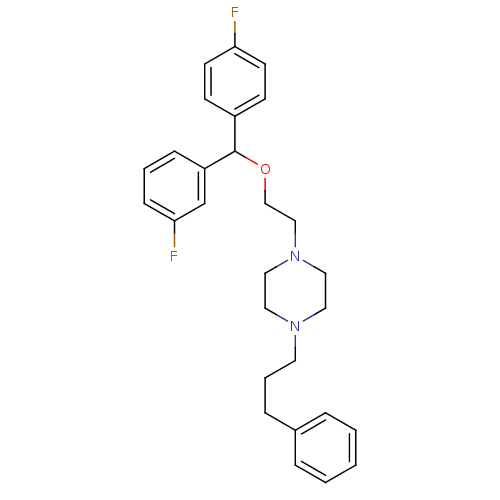 (1-{2-[(3-Fluoro-phenyl)-(4-fluoro-phenyl)-methoxy]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Competitive binding versus [N-methyl-3H]-WIN 35,428 in murine kidney cells transfected with human dopamine transporter | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50085071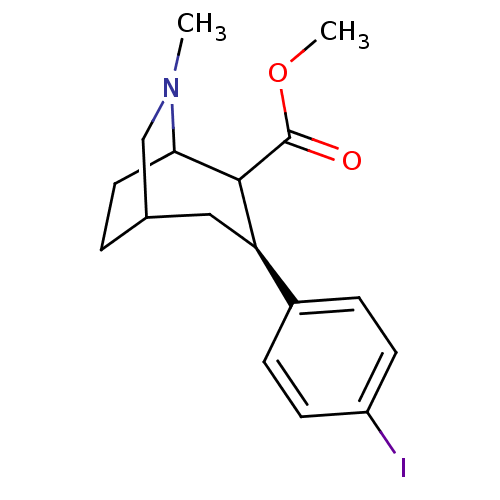 (3-(4-Iodo-phenyl)-6-methyl-6-aza-bicyclo[3.2.2]non...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description In vitro affinity determined using [3H]-WIN- 35428 in murine kidney cells transfected with human dopamine transporter (DAT) | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50085071 (3-(4-Iodo-phenyl)-6-methyl-6-aza-bicyclo[3.2.2]non...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description In vitro affinity determined using [3H]-citalopram in murine kidney cells transfected with human serotonin transporter (SERT) | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50085069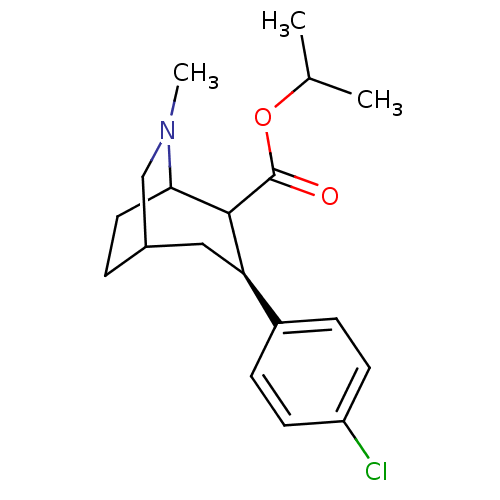 (3-(4-Chloro-phenyl)-6-methyl-6-aza-bicyclo[3.2.2]n...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition constant against [N-methyl-3H]-WIN 35428 in murine kidney cells transfected with human dopamine transporter. | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM85616 (MOLI000038 | [18F]FECNT) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by PDSP Ki Database | Nucl Med Biol 27: 1-12 (2000) Article DOI: 10.1016/s0969-8051(99)00080-3 BindingDB Entry DOI: 10.7270/Q2N29VG7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50028091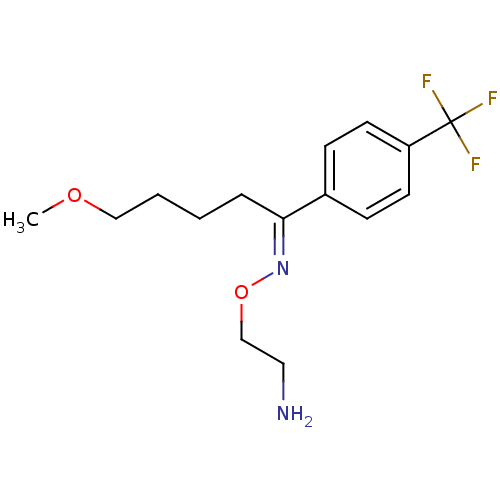 ((1E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition constant against [3H]-citalopram in murine kidney cells transfected with human dopamine transporter | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM85616 (MOLI000038 | [18F]FECNT) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by PDSP Ki Database | Nucl Med Biol 27: 1-12 (2000) Article DOI: 10.1016/s0969-8051(99)00080-3 BindingDB Entry DOI: 10.7270/Q2N29VG7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50085069 (3-(4-Chloro-phenyl)-6-methyl-6-aza-bicyclo[3.2.2]n...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition constant against [3H]-citalopram in murine kidney cells transfected with human serotonin transporter. | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50409556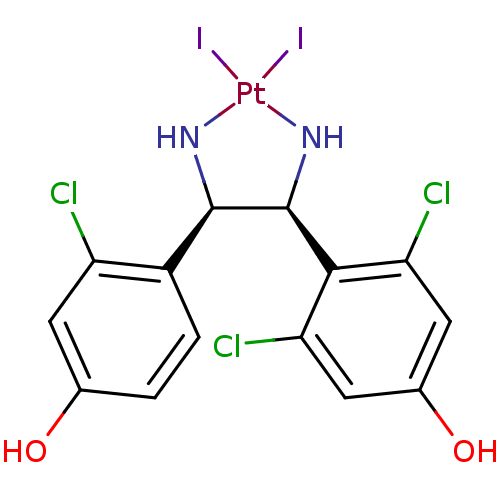 (CHEMBL2068430) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Free University of Berlin Curated by ChEMBL | Assay Description Agonist activity in transcriptional activation assay in MCF-7-2a cells compared to estradiol E2 | J Med Chem 45: 3356-65 (2002) BindingDB Entry DOI: 10.7270/Q29W0GP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50409553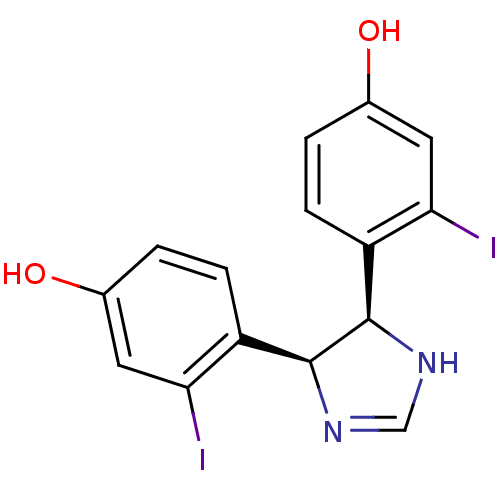 (CHEMBL112355) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Free University of Berlin Curated by ChEMBL | Assay Description Agonist activity in transcriptional activation assay in MCF-7-2a cells compared to estradiol E2 | J Med Chem 45: 3356-65 (2002) BindingDB Entry DOI: 10.7270/Q29W0GP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50409554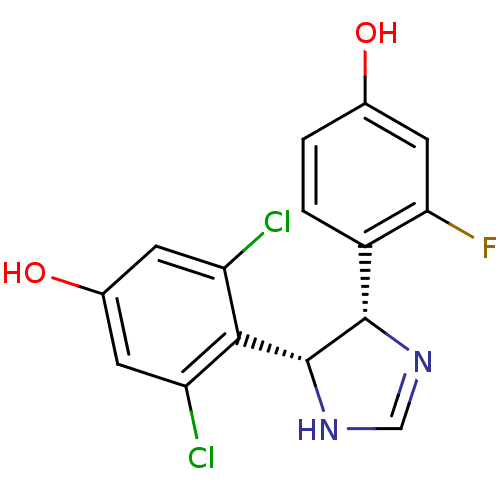 (CHEMBL115729) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a |
Free University of Berlin Curated by ChEMBL | Assay Description Agonist activity in transcriptional activation assay in MCF-7-2a cells compared to estradiol E2 | J Med Chem 45: 3356-65 (2002) BindingDB Entry DOI: 10.7270/Q29W0GP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50116387 (2-Chloro-4-[5-(2-chloro-4-hydroxy-phenyl)-4,5-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Free University of Berlin Curated by ChEMBL | Assay Description Agonist activity in transcriptional activation assay in MCF-7-2a cells compared to estradiol E2 | J Med Chem 45: 3356-65 (2002) BindingDB Entry DOI: 10.7270/Q29W0GP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50409555 (CHEMBL323919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Free University of Berlin Curated by ChEMBL | Assay Description Agonist activity in transcriptional activation assay in MCF-7-2a cells compared to estradiol E2 | J Med Chem 45: 3356-65 (2002) BindingDB Entry DOI: 10.7270/Q29W0GP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
Free University of Berlin Curated by ChEMBL | Assay Description Agonist activity in transcriptional activation assay in MCF-7-2a cells compared to estradiol E2 | J Med Chem 45: 3356-65 (2002) BindingDB Entry DOI: 10.7270/Q29W0GP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||